Endothelial ILK induces cardioprotection by preventing coronary microvascular dysfunction and endothelial-to-mesenchymal transition
- PMID: 37452166
- PMCID: PMC10348984
- DOI: 10.1007/s00395-023-00997-0
Endothelial ILK induces cardioprotection by preventing coronary microvascular dysfunction and endothelial-to-mesenchymal transition
Abstract
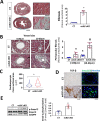
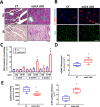
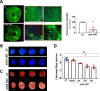
Endothelial dysfunction is an early event in coronary microvascular disease. Integrin-linked kinase (ILK) prevents endothelial nitric oxide synthase (eNOS) uncoupling and, thus, endothelial dysfunction. However, the specific role of endothelial ILK in cardiac function remains to be fully elucidated. We hypothesised that endothelial ILK plays a crucial role in maintaining coronary microvascular function and contractile performance in the heart. We generated an endothelial cell-specific ILK conditional knock-out mouse (ecILK cKO) and investigated cardiovascular function. Coronary endothelial ILK deletion significantly impaired cardiac function: ejection fraction, fractional shortening and cardiac output decreased, whilst left ventricle diastolic internal diameter decreased and E/A and E/E' ratios increased, indicating not only systolic but also diastolic dysfunction. The functional data correlated with extensive extracellular matrix remodelling and perivascular fibrosis, indicative of adverse cardiac remodelling. Mice with endothelial ILK deletion suffered early ischaemic-like events with ST elevation and transient increases in cardiac troponins, which correlated with fibrotic remodelling. In addition, ecILK cKO mice exhibited many features of coronary microvascular disease: reduced cardiac perfusion, impaired coronary flow reserve and arterial remodelling with patent epicardial coronary arteries. Moreover, endothelial ILK deletion induced a moderate increase in blood pressure, but the antihypertensive drug Losartan did not affect microvascular remodelling whilst only partially ameliorated fibrotic remodelling. The plasma miRNA profile reveals endothelial-to-mesenchymal transition (endMT) as an upregulated pathway in endothelial ILK conditional KO mice. Our results show that endothelial cells in the microvasculature in endothelial ILK conditional KO mice underwent endMT. Moreover, endothelial cells isolated from these mice and ILK-silenced human microvascular endothelial cells underwent endMT, indicating that decreased endothelial ILK contributes directly to this endothelial phenotype shift. Our results identify ILK as a crucial regulator of microvascular endothelial homeostasis. Endothelial ILK prevents microvascular dysfunction and cardiac remodelling, contributing to the maintenance of the endothelial cell phenotype.
Keywords: Endothelial to mesenchymal transition; Fibrosis; Integrin-linked kinase; Ischemia; Microvascular dysfunction; Microvessel remodeling.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Chevalier J, Yin H, Arpino JM, O’Neil C, Nong Z, Gilmore KJ, Lee JJ, Prescott E, Hewak M, Rice CL, Dubois L, Power AH, Hamilton DW, Pickering JG. Obstruction of small arterioles in patients with critical limb ischemia due to partial endothelial-to-mesenchymal transition. iScience. 2020 doi: 10.1016/J.ISCI.2020.101251. - DOI - PMC - PubMed
-
- Cuadrado I, Castejon B, Martin AM, Saura M, Reventun-Torralba P, Zamorano JL, Zaragoza C. Nitric oxide induces cardiac protection by preventing extracellular matrix degradation through the complex caveolin-3/emmprin in cardiac myocytes. PLoS ONE. 2016 doi: 10.1371/journal.pone.0162912. - DOI - PMC - PubMed
-
- Cuadrado I, Piedras MJGM, Herruzo I, del Carmen TM, Castejón B, Reventun P, Martin A, Saura M, Zamorano JL, Zaragoza C. EMMPRIN-targeted magnetic nanoparticles for in vivo visualization and regression of acute myocardial infarction. Theranostics. 2016;6:545–557. doi: 10.7150/THNO.13352. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

