Development and multicenter validation of a multiparametric imaging model to predict treatment response in rectal cancer
- PMID: 37452176
- PMCID: PMC10667134
- DOI: 10.1007/s00330-023-09920-6
Development and multicenter validation of a multiparametric imaging model to predict treatment response in rectal cancer
Abstract
Objectives: To develop and validate a multiparametric model to predict neoadjuvant treatment response in rectal cancer at baseline using a heterogeneous multicenter MRI dataset.
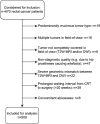
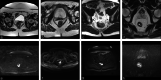
Methods: Baseline staging MRIs (T2W (T2-weighted)-MRI, diffusion-weighted imaging (DWI) / apparent diffusion coefficient (ADC)) of 509 patients (9 centres) treated with neoadjuvant chemoradiotherapy (CRT) were collected. Response was defined as (1) complete versus incomplete response, or (2) good (Mandard tumor regression grade (TRG) 1-2) versus poor response (TRG3-5). Prediction models were developed using combinations of the following variable groups: (1) Non-imaging: age/sex/tumor-location/tumor-morphology/CRT-surgery interval (2) Basic staging: cT-stage/cN-stage/mesorectal fascia involvement, derived from (2a) original staging reports, or (2b) expert re-evaluation (3) Advanced staging: variables from 2b combined with cTN-substaging/invasion depth/extramural vascular invasion/tumor length (4) Quantitative imaging: tumour volume + first-order histogram features (from T2W-MRI and DWI/ADC) Models were developed with data from 6 centers (n = 412) using logistic regression with the Least Absolute Shrinkage and Selector Operator (LASSO) feature selection, internally validated using repeated (n = 100) random hold-out validation, and externally validated using data from 3 centers (n = 97).
Results: After external validation, the best model (including non-imaging and advanced staging variables) achieved an area under the curve of 0.60 (95%CI=0.48-0.72) to predict complete response and 0.65 (95%CI=0.53-0.76) to predict a good response. Quantitative variables did not improve model performance. Basic staging variables consistently achieved lower performance compared to advanced staging variables.
Conclusions: Overall model performance was moderate. Best results were obtained using advanced staging variables, highlighting the importance of good-quality staging according to current guidelines. Quantitative imaging features had no added value (in this heterogeneous dataset).
Clinical relevance statement: Predicting tumour response at baseline could aid in tailoring neoadjuvant therapies for rectal cancer. This study shows that image-based prediction models are promising, though are negatively affected by variations in staging quality and MRI acquisition, urging the need for harmonization.
Key points: This multicenter study combining clinical information and features derived from MRI rendered disappointing performance to predict response to neoadjuvant treatment in rectal cancer. Best results were obtained with the combination of clinical baseline information and state-of-the-art image-based staging variables, highlighting the importance of good quality staging according to current guidelines and staging templates. No added value was found for quantitative imaging features in this multicenter retrospective study. This is likely related to acquisition variations, which is a major problem for feature reproducibility and thus model generalizability.
Keywords: Chemoradiotherapy; Magnetic resonance imaging; Rectal neoplasms.
© 2023. The Author(s).
Conflict of interest statement
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Figures

References
-
- van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391:2537–2545. doi: 10.1016/S0140-6736(18)31078-X. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

