Hepatocyte-specific O-GlcNAc transferase downregulation ameliorates nonalcoholic steatohepatitis by improving mitochondrial function
- PMID: 37453647
- PMCID: PMC10382944
- DOI: 10.1016/j.molmet.2023.101776
Hepatocyte-specific O-GlcNAc transferase downregulation ameliorates nonalcoholic steatohepatitis by improving mitochondrial function
Abstract
Objective: O-GlcNAcylation is a post-translational modification that directly couples the processes of nutrient sensing, metabolism, and signal transduction, affecting protein function and localization, since the O-linked N-acetylglucosamine moiety comes directly from the metabolism of glucose, lipids, and amino acids. The addition and removal of O-GlcNAc of target proteins are mediated by two highly conserved enzymes: O-linked N-acetylglucosamine (O-GlcNAc) transferase (OGT) and O-GlcNAcase (OGA), respectively. Deregulation of O-GlcNAcylation has been reported to be associated with various human diseases such as cancer, diabetes, and cardiovascular diseases. The contribution of deregulated O-GlcNAcylation to the progression and pathogenesis of NAFLD remains intriguing, and a better understanding of its roles in this pathophysiological context is required to uncover novel avenues for therapeutic intervention. By using a translational approach, our aim is to describe the role of OGT and O-GlcNAcylation in the pathogenesis of NAFLD.
Methods: We used primary mouse hepatocytes, human hepatic cell lines and in vivo mouse models of steatohepatitis to manipulate O-GlcNAc transferase (OGT). We also studied OGT and O-GlcNAcylation in liver samples from different cohorts of people with NAFLD.
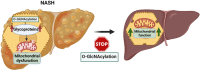
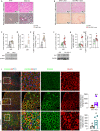
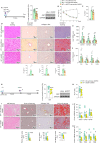
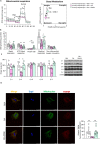
Results: O-GlcNAcylation was upregulated in the liver of people and animal models with steatohepatitis. Downregulation of OGT in NAFLD-hepatocytes improved diet-induced liver injury in both in vivo and in vitro models. Proteomics studies revealed that mitochondrial proteins were hyper-O-GlcNAcylated in the liver of mice with steatohepatitis. Inhibition of OGT is able to restore mitochondrial oxidation and decrease hepatic lipid content in in vitro and in vivo models of NAFLD.
Conclusions: These results demonstrate that deregulated hyper-O-GlcNAcylation favors NAFLD progression by reducing mitochondrial oxidation and promoting hepatic lipid accumulation.
Keywords: Mitochondrial dysfunction; NAFLD; O-GlcNAcylation.
Copyright © 2023 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no conflict of interest
Figures




References
-
- Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. - PubMed
-
- Harrison S.A., Gawrieh S., Roberts K., Lisanti C.J., Schwope R.B., Cebe K.M., et al. Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J Hepatol. 2021;75(2):284–291. - PubMed
-
- Chitturi S., Abeygunasekera S., Farrell G.C., Holmes-Walker J., Hui J.M., Fung C., et al. NASH and insulin resistance: insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35(2):373–379. - PubMed
-
- Targher G., Corey K.E., Byrne C.D., Roden M. The complex link between NAFLD and type 2 diabetes mellitus – mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18(9):599–612. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

