Biventricular Interaction During Acute Left Ventricular Ischemia in Mice: A Combined In-Vivo and In-Silico Approach
- PMID: 37453977
- PMCID: PMC10598180
- DOI: 10.1007/s10439-023-03293-z
Biventricular Interaction During Acute Left Ventricular Ischemia in Mice: A Combined In-Vivo and In-Silico Approach
Abstract
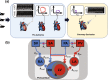
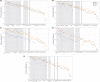
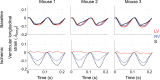
Computational models provide an efficient paradigm for integrating and linking multiple spatial and temporal scales. However, these models are difficult to parameterize and match to experimental data. Recent advances in both data collection and model analyses have helped overcome this limitation. Here, we combine a multiscale, biventricular interaction model with mouse data before and after left ventricular (LV) ischemia. Sensitivity analyses are used to identify the most influential parameters on pressure and volume predictions. The subset of influential model parameters are calibrated to biventricular pressure-volume loop data (n = 3) at baseline. Each mouse underwent left anterior descending coronary artery ligation, during which changes in fractional shortening and RV pressure-volume dynamics were recorded. Using the calibrated model, we simulate acute LV ischemia and contrast outputs at baseline and in simulated ischemia. Our baseline simulations align with the LV and RV data, and our predictions during ischemia complement recorded RV data and prior studies on LV function during myocardial infarction. We show that a model with both biventricular mechanical interaction and systems-level cardiovascular dynamics can quantitatively reproduce in-vivo data and qualitatively match prior findings from animal studies on LV ischemia.
Keywords: Biventricular interaction; Computational model; Multiscale modeling; Myocardial infarction; Parameter estimation; Sensitivity analysis.
© 2023. The Author(s).
Figures





Update of
-
Biventricular interaction during acute left ventricular ischemia in mice: a combined in-vivo and in-silico approach.bioRxiv [Preprint]. 2023 Jan 27:2023.01.26.525736. doi: 10.1101/2023.01.26.525736. bioRxiv. 2023. Update in: Ann Biomed Eng. 2023 Nov;51(11):2528-2543. doi: 10.1007/s10439-023-03293-z. PMID: 36747704 Free PMC article. Updated. Preprint.
Similar articles
-
Biventricular interaction during acute left ventricular ischemia in mice: a combined in-vivo and in-silico approach.bioRxiv [Preprint]. 2023 Jan 27:2023.01.26.525736. doi: 10.1101/2023.01.26.525736. bioRxiv. 2023. Update in: Ann Biomed Eng. 2023 Nov;51(11):2528-2543. doi: 10.1007/s10439-023-03293-z. PMID: 36747704 Free PMC article. Updated. Preprint.
-
Beta-blockers in patients without heart failure after myocardial infarction.Cochrane Database Syst Rev. 2021 Nov 5;11(11):CD012565. doi: 10.1002/14651858.CD012565.pub2. Cochrane Database Syst Rev. 2021. PMID: 34739733 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Magnetic resonance perfusion for differentiating low-grade from high-grade gliomas at first presentation.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD011551. doi: 10.1002/14651858.CD011551.pub2. Cochrane Database Syst Rev. 2018. PMID: 29357120 Free PMC article.
-
Simulated Effects of Acute Left Ventricular Myocardial Infarction on Mitral Regurgitation in an Ovine Model.J Biomech Eng. 2024 Oct 1;146(10):101009. doi: 10.1115/1.4065376. J Biomech Eng. 2024. PMID: 38652602
Cited by
-
Guidelines for mechanistic modeling and analysis in cardiovascular research.Am J Physiol Heart Circ Physiol. 2024 Aug 1;327(2):H473-H503. doi: 10.1152/ajpheart.00766.2023. Epub 2024 Jun 21. Am J Physiol Heart Circ Physiol. 2024. PMID: 38904851 Free PMC article. Review.
-
The right ventricular dysfunction and ventricular interdependence in patients with T2DM and aortic regurgitation: an assessment using CMR feature tracking.Cardiovasc Diabetol. 2024 Aug 8;23(1):294. doi: 10.1186/s12933-024-02372-2. Cardiovasc Diabetol. 2024. PMID: 39118075 Free PMC article.
References
-
- Allen B, Frye H, Ramanathan R, Caggiano LR, Tabima DM, Chesler NC, Philip JL. Biomechanical and mechanobiological drivers of the transition from postcapillary pulmonary hypertension to combined pre−/postcapillary pulmonary hypertension. J. Am. Heart Assoc. 2023;12:1–15. doi: 10.1161/JAHA.122.028121. - DOI - PMC - PubMed
-
- Banks HT, Hu S, Thompson WC. Modeling and Inverse Problems in the Presence of Uncertainty. New York: Chapman and Hall/CRC; 2014.
-
- Bauer F, Jones M, Jian XQ, Castro P, Asada J, Sitges M, Cardon LA, Tsujino H, Zetts AD, Panza JA, Thomas JD, Shiota T. Quantitative analysis of left atrial function during left ventricular ischemia with and without left atrial ischemia: a real-time 3-dimensional echocardiographic study. J. Am. Soc. Echocardiogr. 2005;18:795–801. doi: 10.1016/j.echo.2005.01.018. - DOI - PubMed

