Chromatographic reversed HPLC and TLC-densitometry methods for simultaneous determination of serdexmethylphenidate and dexmethylphenidate in presence of their degradation products-with computational assessment
- PMID: 37454105
- PMCID: PMC10349413
- DOI: 10.1186/s13065-023-00986-3
Chromatographic reversed HPLC and TLC-densitometry methods for simultaneous determination of serdexmethylphenidate and dexmethylphenidate in presence of their degradation products-with computational assessment
Abstract
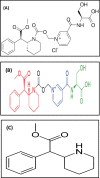
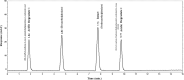
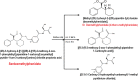
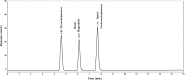
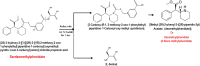
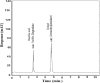
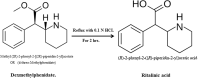
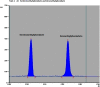
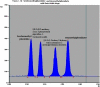
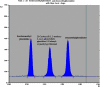
Two Chromatographic methods have been established and optimized for simultaneous determination of serdexmethylphenidate (SER.DMP) and dexmethylphenidate (DMP) in the presence of their degradation products. The first method is a reversed phase high performance liquid chromatography with diode array detection (HPLC-DAD). Isocratic separation was carried out on Waters X-bridge Shield RP18 column (150×3.9×5 μm particle size) using a mixture of 5 mM phosphate buffer (pH 5.5): acetonitrile (40:60, v/v) as a mobile phase, flow rate 1 mL/min and detection at 220 nm. The second method is a thin-layer chromatography (TLC)-densitometry method using methanol: chloroform (70:30, v/v) as a mobile phase and UV scanning at 220 nm. In HPLC method, the linearity range of SER.DMP was (2.5-25 μg/mL); with LOD (0.051 μg/mL) and LOQ (0.165 μg/mL) while for DMP was (2.5-25 μg/mL); with LOD and LOQ of (0.098 μg/mL) and (0.186 μg/mL), respectively. For TLC method the sensitivity range of SER.DMP was (5-25 μg/mL), LOD was (0.184 μg/spot), while LOQ was (0.202 μg/ spot) whereas for DMP the sensitivity range was (5-25 μg/mL) with LOD of (0.115 μg/ spot) and LOQ of (0.237 μg/ spot), respectively. SER.DMP was found to be equally labile to acidic and alkaline hydrolysis, whereas DMP was sensitive to acidic hydrolysis only. Both drugs were successfully determined in presence of acidic and basic degradants by the two developed methods (stability indicating assay method). Chromatographic separation of the degradation products was carried out on TLC aluminum silica plates 60 F254, as a stationary phase, using methanol: dichloroethane: acetonitrile (60:20:20 v/v), as a mobile phase. The degradation pathway was confirmed using TLC, IR, 1H-NMR and mass spectroscopy; moreover, the separation power was correlated to the computational results by applying molecular dynamic simulation. The developed methods were validated according to the International Conference on Harmonization (ICH) guidelines demonstrating good accuracy and precision. They were successfully applied for quantitation of SER.DMP and DMP in pure and capsule forms. The results were statistically compared with those obtained by the reported method in terms of accuracy, precision and robustness, and no significant difference was found.
Keywords: Dexmethylphenidate (DMP); HPLC–DAD; Molecular dynamic simulation; SER.DMP and DMP degradation products; Serdexmethylphenidate (SER.DMP); TLC-densitometry.
© 2023. The Author(s).
Conflict of interest statement
There is no conflict of interest to declare.
Figures
Similar articles
-
Simultaneous Determination of Cinchocaine Hydrochloride and Betamethasone Valerate in Presence of Their Degradation Products.J Chromatogr Sci. 2017 May 1;55(5):518-527. doi: 10.1093/chromsci/bmx004. J Chromatogr Sci. 2017. PMID: 28168304
-
Quality and Stability Profile Assessment of the Recent Antidiabetic Omarigliptin by Using Different Chromatographic Methods.J Chromatogr Sci. 2021 Aug 18;59(8):762-769. doi: 10.1093/chromsci/bmaa136. J Chromatogr Sci. 2021. PMID: 33434917
-
Stability-Indicating TLC-Densitometric and HPLC Methods for the Simultaneous Determination of Piracetam and Vincamine in the Presence of Their Degradation Products.J AOAC Int. 2016 Nov 1;99(6):1490-1498. doi: 10.5740/jaoacint.16-0164. Epub 2016 Aug 27. J AOAC Int. 2016. PMID: 27569579
-
Simultaneous analysis of the of levamisole with triclabendazole in pharmaceuticals through developing TLC and HPLC-PDA chromatographic techniques and their greenness assessment using GAPI and AGREE methods.BMC Chem. 2023 Nov 24;17(1):163. doi: 10.1186/s13065-023-01087-x. BMC Chem. 2023. PMID: 37996961 Free PMC article.
-
Stability-Indicating Determination of Tedizolid Phosphate in the Presence of its Active Form and Possible Degradants.J Chromatogr Sci. 2022 Jan 1;60(1):51-60. doi: 10.1093/chromsci/bmab045. J Chromatogr Sci. 2022. PMID: 33881135
Cited by
-
Structural Characterization and Bioactivity Evaluation of Selenium-Modified Dihydromyricetin from Vine Tea.Foods. 2025 May 13;14(10):1735. doi: 10.3390/foods14101735. Foods. 2025. PMID: 40428515 Free PMC article.
References
-
- Sweetman S. Martindale: the complete drug reference. 36. London: The Pharmaceutical Press; 2009.
-
- Pharmacopeia U.S. National Formulary 35, United States Pharmacopeia Convention Inc. 30th edn, 2012–2015.
-
- Center for Drug Evaluation and Research. Drug approval package: AZSTARYS (serdexmethylphenidate and dexmethylphenidate). Silver Spring (MD): FDA; 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/212994Orig1s000T.... Accessed from 18 Nov 2021.
-
- Shram MJ, Setnik B, Webster L, Guenther S, Mickle TC, Braeckman R, Kanski J, Martin A, Kelsh D, Vince BD, Barrett AC. Oral, intranasal, and intravenous abuse potential of serdexmethylphenidate, a novel prodrug of d-methylphenidate. Curr Med Res Opin. 2022;38(7):1237–1250. doi: 10.1080/03007995.2022.2076474. - DOI - PubMed
LinkOut - more resources
Full Text Sources
