VEGFR3 is required for button junction formation in lymphatic vessels
- PMID: 37454290
- PMCID: PMC10503778
- DOI: 10.1016/j.celrep.2023.112777
VEGFR3 is required for button junction formation in lymphatic vessels
Abstract
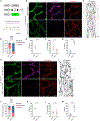
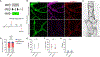
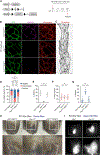
Lymphatic capillaries develop discontinuous cell-cell junctions that permit the absorption of large macromolecules, chylomicrons, and fluid from the interstitium. While excessive vascular endothelial growth factor 2 (VEGFR2) signaling can remodel and seal these junctions, whether and how VEGFR3 can alter lymphatic junctions remains incompletely understood. Here, we use lymphatic-specific Flt4 knockout mice to investigate VEGFR3 signaling in lymphatic junctions. We show that loss of Flt4 prevents specialized button junction formation in multiple tissues and impairs interstitial absorption. Knockdown of FLT4 in human lymphatic endothelial cells results in impaired NOTCH1 expression and activation, and overexpression of the NOTCH1 intracellular domain in Flt4 knockout vessels rescues the formation of button junctions and absorption of interstitial molecules. Together, our data reveal a requirement for VEGFR3 and NOTCH1 signaling in the development of button junctions during postnatal development and may hold clinical relevance to lymphatic diseases with impaired VEGFR3 signaling.
Keywords: CP: Developmental biology; FLT4; Milroy; NOTCH; VEGFR3; button; junctions; lymphatic; lymphedema; zipper.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

