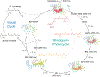
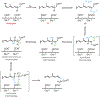
A short story on how chromophore is hydrolyzed from rhodopsin for recycling
- PMID: 37454357
- PMCID: PMC10614701
- DOI: 10.1002/bies.202300068
A short story on how chromophore is hydrolyzed from rhodopsin for recycling
Abstract
The photocycle of visual opsins is essential to maintain the light sensitivity of the retina. The early physical observations of the rhodopsin photocycle by Böll and Kühne in the 1870s inspired over a century's worth of investigations on rhodopsin biochemistry. A single photon isomerizes the Schiff-base linked 11-cis-retinylidene chromophore of rhodopsin, converting it to the all-trans agonist to elicit phototransduction through photoactivated rhodopsin (Rho*). Schiff base hydrolysis of the agonist is a key step in the photocycle, not only diminishing ongoing phototransduction but also allowing for entry and binding of fresh 11-cis chromophore to regenerate the rhodopsin pigment and maintain light sensitivity. Many challenges have been encountered in measuring the rate of this hydrolysis, but recent advancements have facilitated studies of the hydrolysis within the native membrane environment of rhodopsin. These techniques can now be applied to study hydrolysis of agonist in other opsin proteins that mediate phototransduction or chromophore turnover. In this review, we discuss the progress that has been made in characterizing the rhodopsin photocycle and the journey to characterize the hydrolysis of its all-trans-retinylidene agonist.
Keywords: 11-cis-retinal; all-trans-retinal; chromophore; dark adaptation; retinal hydrolysis; retinylidene phospholipids; rhodopsin.
© 2023 The Authors. BioEssays published by Wiley Periodicals LLC.
Conflict of interest statement
Figures