Genetic deletion of c-Jun amino-terminal kinase 3 (JNK3) modestly increases disease severity in a mouse model of multiple sclerosis
- PMID: 37454525
- PMCID: PMC10527920
- DOI: 10.1016/j.jneuroim.2023.578152
Genetic deletion of c-Jun amino-terminal kinase 3 (JNK3) modestly increases disease severity in a mouse model of multiple sclerosis
Abstract
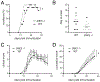
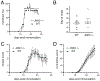
The c-Jun amino terminal kinases (JNKs) regulate transcription, and studies suggest they contribute to neuropathology in the EAE model of MS. To examine the role of the JNK3 isoform, we compared EAE in JNK3 null mice to wild type (WT) littermates. Although disease severity was similar in female mice, in male JNK3 null mice the day of onset and time to reach 100% incidence occurred sooner, and disease severity was increased. While glial activation in spinal cord was similar, white matter lesions were increased in JNK3 null mice. These results suggest JNK3 normally limits EAE disease in a sex-dependent manner.
Keywords: Astrocytes; EAE; JNK3; Microglia; Multiple sclerosis; Spinal cord.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest None.
Figures
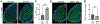
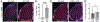

References
-
- Barnat M, Enslen H, Propst F, Davis RJ, Soares S, Nothias F. Distinct roles of c-Jun N-terminal kinase isoforms in neurite initiation and elongation during axonal regeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:7804–16. 10.1523/j.neurosci.0372-10.2010. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

