Antioxidant mitoquinone suppresses benign prostatic hyperplasia by regulating the AR-NLRP3 pathway
- PMID: 37454529
- PMCID: PMC10368918
- DOI: 10.1016/j.redox.2023.102816
Antioxidant mitoquinone suppresses benign prostatic hyperplasia by regulating the AR-NLRP3 pathway
Abstract
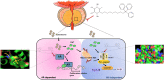
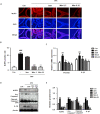
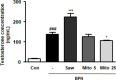
Mitoquinone (MitoQ), a mitochondria-targeted antioxidant, has been used to treat several diseases. The present study aimed to investigate the therapeutic effects of MitoQ in benign prostatic hyperplasia (BPH) models and their underlying molecular mechanisms. In this study, we determined that MitoQ inhibited dihydrotestosterone (DHT)-induced cell proliferation and mitochondrial ROS by inhibiting androgen receptor (AR) and NOD-like receptor family pyrin domain-containing 3 (NLRP3) signaling in prostate epithelial cells. Molecular modeling revealed that DHT may combine with AR and NLRP3, and that MitoQ inhibits both AR and NLRP3. AR and NLRP3 downregulation using siRNA showed the linkage among AR, NLRP3, and MitoQ. MitoQ administration alleviated pathological prostate enlargement and exerted anti-proliferative and antioxidant effects by suppressing the AR and NLRP3 signaling pathways in rats with BPH. Hence, our findings demonstrated that MitoQ is an inhibitor of NLPR3 and AR and a therapeutic agent for BPH treatment.
Keywords: Androgen receptor; Benign prostatic hyperplasia; Dihydrotestosterone; Mitoquinone; NLRP3.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Roehrborn C.G. Male lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH) Med. Clin. 2011 Jan;95(1):87–100. PubMed PMID: 21095413. - PubMed
-
- Glynn R.J., Campion E.W., Bouchard G.R., Silbert J.E. The development of benign prostatic hyperplasia among volunteers in the Normative Aging Study. Am. J. Epidemiol. 1985 Jan;121(1):78–90. PubMed PMID: 3964994. - PubMed
-
- Naslund M.J., Gilsenan A.W., Midkiff K.D., Bown A., Wolford E.T., Wang J. Prevalence of lower urinary tract symptoms and prostate enlargement in the primary care setting. Int. J. Clin. Pract. 2007 Sep;61(9):1437–1445. PubMed PMID: 17686091. - PubMed
-
- Awedew A.F., Han H., Abbasi B., Abbasi-Kangevari M., Ahmed M.B., Almidani O., et al. The global, regional, and national burden of benign prostatic hyperplasia in 204 countries and territories from 2000 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet Healthy Longevity. 2022;3(11):e754–e776. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

