Alterations of hepatic energy metabolism in murine models of obesity, diabetes and fatty liver diseases
- PMID: 37454552
- PMCID: PMC10384226
- DOI: 10.1016/j.ebiom.2023.104714
Alterations of hepatic energy metabolism in murine models of obesity, diabetes and fatty liver diseases
Abstract
Background: Disturbed hepatic energy metabolism contributes to non-alcoholic fatty liver (NAFLD), but the development of changes over time and obesity- or diabetes-related mechanisms remained unclear.
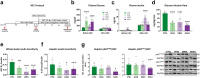
Methods: Two-day old male C57BL/6j mice received streptozotocin (STZ) or placebo (PLC) and then high-fat (HFD) or regular chow diet (RCD) from week 4 (W4) to either W8 or W16, yielding control [CTRL = PLC + RCD], diabetes [DIAB = STZ + RCD], obesity [OBES = PLC + HFD] and diabetes-related non-alcoholic steatohepatitis [NASH = STZ + HFD] models. Mitochondrial respiration was measured by high-resolution respirometry and insulin-sensitive glucose metabolism by hyperinsulinemic-euglycemic clamps with stable isotope dilution.
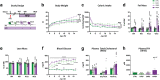
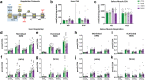
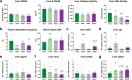
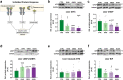
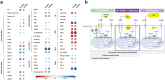
Findings: NASH showed higher steatosis and NAFLD activity already at W8 and liver fibrosis at W16 (all p < 0.01 vs CTRL). Ballooning was increased in DIAB and NASH at W16 (p < 0.01 vs CTRL). At W16, insulin sensitivity was 47%, 58% and 75% lower in DIAB, NASH and OBES (p < 0.001 vs CTRL). Hepatic uncoupled fatty acid oxidation (FAO)-associated respiration was reduced in OBES at W8, but doubled in DIAB and NASH at W16 (p < 0.01 vs CTRL) and correlated with biomarkers of unfolded protein response (UPR), oxidative stress and hepatic expression of certain enzymes (acetyl-CoA carboxylase 2, Acc2; carnitine palmitoyltransferase I, Cpt1a). Tricarboxylic acid cycle (TCA)-driven respiration was lower in OBES at W8 and doubled in DIAB at W16 (p < 0.0001 vs CTRL), which positively correlated with expression of genes related to lipolysis.
Interpretation: Hepatic mitochondria adapt to various metabolic challenges with increasing FAO-driven respiration, which is linked to dysfunctional UPR, systemic oxidative stress, insulin resistance and altered lipid metabolism. In a diabetes model, higher TCA-linked respiration reflected mitochondrial adaptation to greater hepatic lipid turnover.
Funding: Funding bodies that contributed to this study were listed in the acknowledgements section.
Keywords: Fatty liver; High-resolution respirometry; Insulin resistance; Mitochondria; Type 1 diabetes; Type 2 diabetes; Unfolded protein response.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests LM, CE, CR, CG, ER, DP, MW, IE, MDF, FZ, and AY declare no competing interests. BD is supported by a DZD grant. GH is supported by the Jühling Award. MR is currently on scientific advisory boards of Astra Zeneca, Boehringer Ingelheim, Eli Lilly, NovoNordisk, and Target RWE, and has received investigator-initiated support from Boehringer Ingelheim, Nutricia/Danone and Sanofi-Aventis.
Figures

References
-
- Chalasani N., Younossi Z., Lavine J.E., et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the study of liver diseases. Hepatology. 2018;67:328–357. - PubMed
-
- European Association for the Study of the Liver (EASL) European Association for the study of diabetes (EASD), European Association for the study of obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. - PubMed
-
- Mansouri A., Gattolliat C.-H., Asselah T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology. 2018;155:629–647. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

