HLA-DP on Epithelial Cells Enables Tissue Damage by NKp44+ Natural Killer Cells in Ulcerative Colitis
- PMID: 37454979
- PMCID: PMC10529779
- DOI: 10.1053/j.gastro.2023.06.034
HLA-DP on Epithelial Cells Enables Tissue Damage by NKp44+ Natural Killer Cells in Ulcerative Colitis
Abstract
Background & aims: Ulcerative colitis (UC) is characterized by severe inflammation and destruction of the intestinal epithelium, and is associated with specific risk single nucleotide polymorphisms in HLA class II. Given the recently discovered interactions between subsets of HLA-DP molecules and the activating natural killer (NK) cell receptor NKp44, genetic associations of UC and HLA-DP haplotypes and their functional implications were investigated.
Methods: HLA-DP haplotype and UC risk association analyses were performed (UC: n = 13,927; control: n = 26,764). Expression levels of HLA-DP on intestinal epithelial cells (IECs) in individuals with and without UC were quantified. Human intestinal 3-dimensional (3D) organoid cocultures with human NK cells were used to determine functional consequences of interactions between HLA-DP and NKp44.
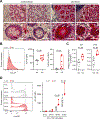
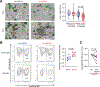
Results: These studies identified HLA-DPA1∗01:03-DPB1∗04:01 (HLA-DP401) as a risk haplotype and HLA-DPA1∗01:03-DPB1∗03:01 (HLA-DP301) as a protective haplotype for UC in European populations. HLA-DP expression was significantly higher on IECs of individuals with UC compared with controls. IECs in human intestinal 3D organoids derived from HLA-DP401pos individuals showed significantly stronger binding of NKp44 compared with HLA-DP301pos IECs. HLA-DP401pos IECs in organoids triggered increased degranulation and tumor necrosis factor production by NKp44+ NK cells in cocultures, resulting in enhanced epithelial cell death compared with HLA-DP301pos organoids. Blocking of HLA-DP401-NKp44 interactions (anti-NKp44) abrogated NK cell activity in cocultures.
Conclusions: We identified an UC risk HLA-DP haplotype that engages NKp44 and activates NKp44+ NK cells, mediating damage to intestinal epithelial cells in an HLA-DP haplotype-dependent manner. The molecular interaction between NKp44 and HLA-DP401 in UC can be targeted by therapeutic interventions to reduce NKp44+ NK cell-mediated destruction of the intestinal epithelium in UC.
Keywords: HLA-DP; Intestinal Organoids; NK Cells; NKp44; Ulcerative Colitis.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
A.N. and M.A. are inventors of one provisional patent that describes the binding of NKp44 to a subset of HLA-DP molecules, and the role of these interactions in Graft versus Host Disease. All other authors declare no conflicts of interest.
Figures



References
-
- Boer NKH de, Ahuja V, Almer S, et al. Thiopurine Therapy in Inflammatory Bowel Diseases: Making New Friends Should Not Mean Losing Old Ones. Gastroenterology 2019;156. - PubMed
-
- Al-Horani R, Spanudakis E, Hamad B. The market for ulcerative colitis. Nat Rev Drug Discov 2022;21. - PubMed
-
- Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014;146. - PubMed
-
- Kobayashi T, Siegmund B, Berre C Le, et al. Ulcerative colitis. Nat Rev Dis Prim 2020;6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

