Scutellarin prevents acute alcohol-induced liver injury via inhibiting oxidative stress by regulating the Nrf2/HO-1 pathway and inhibiting inflammation by regulating the AKT, p38 MAPK/NF-κB pathways
- PMID: 37455138
- PMCID: PMC10350365
- DOI: 10.1631/jzus.B2200612
Scutellarin prevents acute alcohol-induced liver injury via inhibiting oxidative stress by regulating the Nrf2/HO-1 pathway and inhibiting inflammation by regulating the AKT, p38 MAPK/NF-κB pathways
Abstract
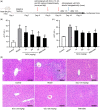
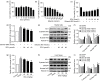
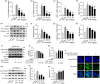
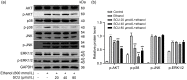
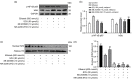
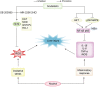
Alcoholic liver disease (ALD) is the most frequent liver disease worldwide, resulting in severe harm to personal health and posing a serious burden to public health. Based on the reported antioxidant and anti-inflammatory capacities of scutellarin (SCU), this study investigated its protective role in male BALB/c mice with acute alcoholic liver injury after oral administration (10, 25, and 50 mg/kg). The results indicated that SCU could lessen serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels and improve the histopathological changes in acute alcoholic liver; it reduced alcohol-induced malondialdehyde (MDA) content and increased glutathione peroxidase (GSH-Px), catalase (CAT), and superoxide dismutase (SOD) activity. Furthermore, SCU decreased tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-1β messenger RNA (mRNA) expression levels, weakened inducible nitric oxide synthase (iNOS) activity, and inhibited nucleotide-binding oligomerization domain (NOD)-like receptor protein 3 (NLRP3) inflammasome activation. Mechanistically, SCU suppressed cytochrome P450 family 2 subfamily E member 1 (CYP2E1) upregulation triggered by alcohol, increased the expression of oxidative stress-related nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) pathways, and suppressed the inflammation-related degradation of inhibitor of nuclear factor-κB (NF-κB)-α (IκBα) as well as activation of NF-κB by mediating the protein kinase B (AKT) and p38 mitogen-activated protein kinase (MAPK) pathways. These findings demonstrate that SCU protects against acute alcoholic liver injury via inhibiting oxidative stress by regulating the Nrf2/HO-1 pathway and suppressing inflammation by regulating the AKT, p38 MAPK/NF-κB pathways.
酒精性肝病(ALD)是世界上最常见的肝脏疾病,严重危害个人健康,对公共卫生造成严重负担。基于灯盏花乙素(SCU)抗氧化和抗炎能力的报道,本研究探究了SCU(10、25和50 mg/kg,口服给药)对急性酒精性肝损伤BALB/c小鼠的保护作用。结果表明:SCU可降低血清谷丙转氨酶(ALT)和天冬氨酸转氨酶(AST)水平,改善急性酒精性肝组织病理改变;降低酒精诱导的丙二醛(MDA)含量,提高谷胱甘肽过氧化物酶(GSH-Px)、过氧化氢酶(CAT)和超氧化物歧化酶(SOD)活性。此外,SCU会降低肿瘤坏死因子-α(TNF-α)、白细胞介素6(IL-6)和IL-1β的mRNA表达水平,削弱诱导型一氧化氮合酶(iNOS)活性和抑制NOD样受体蛋白3(NLRP3)炎症小体激活。从机制方面而言,SCU可抑制酒精诱导的CYP450代谢酶家族中的CYP2E1上调,增加氧化应激相关的核因子E2相关因子2(Nrf2)和改为血红素加氧酶-1(HO-1)通路的表达,通过介导蛋白激酶B(AKT)和p38 MAPK通路抑制炎症相关核因子-κB抑制蛋白α因子的降解以及核因子-κB因子的激活。这些结果表明,SCU通过调控Nrf2/HO-1通路抑制氧化应激,通过调控AKT、p38 MAPK/NF-κB通路抑制炎症反应,从而保护急性酒精性肝损伤。.
酒精性肝病(ALD)是世界上最常见的肝脏疾病,严重危害个人健康,对公共卫生造成严重负担。基于灯盏花乙素(SCU)抗氧化和抗炎能力的报道,本研究探究了SCU(10、25和50 mg/kg,口服给药)对急性酒精性肝损伤BALB/c小鼠的保护作用。结果表明:SCU可降低血清谷丙转氨酶(ALT)和天冬氨酸转氨酶(AST)水平,改善急性酒精性肝组织病理改变;降低酒精诱导的丙二醛(MDA)含量,提高谷胱甘肽过氧化物酶(GSH-Px)、过氧化氢酶(CAT)和超氧化物歧化酶(SOD)活性。此外,SCU会降低肿瘤坏死因子-α(TNF-α)、白细胞介素6(IL-6)和IL-1β的mRNA表达水平,削弱诱导型一氧化氮合酶(iNOS)活性和抑制NOD样受体蛋白3(NLRP3)炎症小体激活。从机制方面而言,SCU可抑制酒精诱导的CYP450代谢酶家族中的CYP2E1上调,增加氧化应激相关的核因子E2相关因子2(Nrf2)和改为血红素加氧酶-1(HO-1)通路的表达,通过介导蛋白激酶B(AKT)和p38 MAPK通路抑制炎症相关核因子-κB抑制蛋白α因子的降解以及核因子-κB因子的激活。这些结果表明,SCU通过调控Nrf2/HO-1通路抑制氧化应激,通过调控AKT、p38 MAPK/NF-κB通路抑制炎症反应,从而保护急性酒精性肝损伤。
Keywords: Alcoholic liver disease; Inflammation; Oxidative stress; Scutellarin.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

