Histone H3 lysine 27 trimethylation suppresses jasmonate biosynthesis and signaling to affect male fertility under high temperature in cotton
- PMID: 37455428
- PMCID: PMC10721488
- DOI: 10.1016/j.xplc.2023.100660
Histone H3 lysine 27 trimethylation suppresses jasmonate biosynthesis and signaling to affect male fertility under high temperature in cotton
Abstract
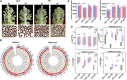
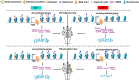
High-temperature (HT) stress causes male sterility in crops, thus decreasing yields. To explore the possible contribution of histone modifications to male fertility under HT conditions, we defined the histone methylation landscape for the marks histone H3 lysine 27 trimethylation (H3K27me3) and histone H3 lysine 4 trimethylation (H3K4me3) by chromatin immunoprecipitation sequencing (ChIP-seq) in two differing upland cotton (Gossypium hirsutum) varieties. We observed a global disruption in H3K4me3 and H3K27me3 modifications, especially H3K27me3, in cotton anthers subjected to HT. HT affected the bivalent H3K4me3-H3K27me3 modification more than either monovalent modification. We determined that removal of H3K27me3 at the promoters of jasmonate-related genes increased their expression, maintaining male fertility under HT in the HT-tolerant variety at the anther dehiscence stage. Modulating jasmonate homeostasis or signaling resulted in an anther indehiscence phenotype under HT. Chemical suppression of H3K27me3 deposition increased jasmonic acid contents and maintained male fertility under HT. In summary, our study provides new insights into the regulation of male fertility by histone modifications under HT and suggests a potential strategy for improving cotton HT tolerance.
Keywords: H3K27me3; H3K4me3; cotton anther; high temperature; jasmonate.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Allis C.D., Jenuwein T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 2016;17:487–500. - PubMed
-
- Azuara V., Perry P., Sauer S., Spivakov M., Jørgensen H.F., John R.M., Gouti M., Casanova M., Warnes G., Merkenschlager M., et al. Chromatin signatures of pluripotent cell lines. Nat. Cell Biol. 2006;8:532–538. - PubMed
-
- Bernstein B.E., Mikkelsen T.S., Xie X., Kamal M., Huebert D.J., Cuff J., Fry B., Meissner A., Wernig M., Plath K., et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

