Plasma Myokine Profiles in Patients With AChR- and MuSK-Ab-Positive Myasthenia Gravis
- PMID: 37455510
- PMCID: PMC10471556
- DOI: 10.3988/jcn.2022.0265
Plasma Myokine Profiles in Patients With AChR- and MuSK-Ab-Positive Myasthenia Gravis
Abstract
Background and purpose: Myokines include cytokines secreted by muscle fibers, which are the final targets of myasthenia gravis (MG). This pilot study investigated whether myokine plasma concentrations are altered in patients with MG and assessed the association between the concentration of each myokine and disease severity.
Methods: We compared the plasma concentrations of 15 myokines in 63 patients with acetylcholine receptor antibody (Ab)-positive MG and 14 with muscle-specific tyrosine kinase Ab-positive MG (MuSK MG) with those in 15 healthy controls. Plasma myokine concentrations were measured using a Luminex multiplex assay kit with magnetic beads that contained Abs for 15 myokines. Correlations between myokine concentration and clinical scale results were analyzed.
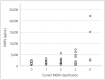
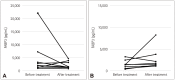
Results: The concentration of fractalkine in plasma was higher in MG (median [interquartile range]=419.6 [38.7-732.5] pg/mL) than in controls (158.5 [0.0-313.2] pg/mL, p=0.034). The leukemia inhibitory factor concentration was also found to be higher in MuSK MG (29.9 [8.7-40.1] pg/mL) than in healthy controls (7.6 [0.0-15.6] pg/mL, p=0.013). Fatty-acid-binding protein 3 (FABP3) concentrations in plasma were positively associated with clinical parameters for MG severity, including scores on the Quantitative Myasthenia Gravis score (p=0.008), Myasthenia Gravis Activities of Daily Living (p=0.003), and Myasthenia Gravis Composite (p=0.024) scales. FABP3 concentration in plasma tended to decrease after treatment in patients without additional relapse but increased in those with further relapse.
Conclusions: The plasma myokine profile was significantly altered in patients with MG. FABP3 concentration may be useful in assessing disease severity and predicting the treatment response.
Keywords: cytokine; myasthenia gravis; myokine.
Copyright © 2023 Korean Neurological Association.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures


Similar articles
-
Clinical Features and Prognostic Analysis of MuSK-Antibody-Positive Myasthenia Gravis versus Double-Seropositive Myasthenia Gravis: A Single-Center Study from Central South China.Neuropsychiatr Dis Treat. 2024 Mar 29;20:725-735. doi: 10.2147/NDT.S450651. eCollection 2024. Neuropsychiatr Dis Treat. 2024. PMID: 38566882 Free PMC article.
-
Association between musk antibody concentrations and the myasthenia gravis composite score in 3 patients: A marker of relapse?Muscle Nerve. 2019 Sep;60(3):307-311. doi: 10.1002/mus.26609. Epub 2019 Jun 25. Muscle Nerve. 2019. PMID: 31177576
-
Thymus histology and concomitant autoimmune diseases in Japanese patients with muscle-specific receptor tyrosine kinase-antibody-positive myasthenia gravis.Eur J Neurol. 2013 Sep;20(9):1272-6. doi: 10.1111/ene.12169. Epub 2013 May 17. Eur J Neurol. 2013. PMID: 23679930
-
AChR myasthenia gravis switching to MuSK or double antibody positive myasthenia gravis in two children and literature review.Neuromuscul Disord. 2020 Jul;30(7):534-538. doi: 10.1016/j.nmd.2020.03.012. Epub 2020 Apr 13. Neuromuscul Disord. 2020. PMID: 32387283 Review.
-
Neuromuscular junction autoimmune disease: muscle specific kinase antibodies and treatments for myasthenia gravis.Curr Opin Neurol. 2005 Oct;18(5):519-25. doi: 10.1097/01.wco.0000180660.57801.3f. Curr Opin Neurol. 2005. PMID: 16155434 Review.
Cited by
-
Circulating inflammatory proteins as pathogenic mediators and potential therapeutic targets in myasthenia gravis.Neurol Sci. 2025 Jun 26. doi: 10.1007/s10072-025-08328-y. Online ahead of print. Neurol Sci. 2025. PMID: 40569533
References
-
- Feferman T, Maiti PK, Berrih-Aknin S, Bismuth J, Bidault J, Fuchs S, et al. Overexpression of IFN-induced protein 10 and its receptor CXCR3 in myasthenia gravis. J Immunol. 2005;174:5324–5331. - PubMed
-
- Garcia YR, May JJ, Green AM, Krolick KA. Acetylcholine receptor-reactive antibody induces nitric oxide production by a rat skeletal muscle cell line: influence of cytokine environment. J Neuroimmunol. 2001;120:103–111. - PubMed
-
- Li H, Shi FD, Bai X, Huang Y, Diab A, He B, et al. Cytokine and chemokine mRNA expressing cells in muscle tissues of experimental autoimmune myasthenia gravis. J Neurol Sci. 1998;161:40–46. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

