Targeted volume correlative light and electron microscopy of an environmental marine microorganism
- PMID: 37455654
- PMCID: PMC10445747
- DOI: 10.1242/jcs.261355
Targeted volume correlative light and electron microscopy of an environmental marine microorganism
Abstract
Photosynthetic microalgae are responsible for an important fraction of CO2 fixation and O2 production on Earth. Three-dimensional (3D) ultrastructural characterization of these organisms in their natural environment can contribute to a deeper understanding of their cell biology. However, the low throughput of volume electron microscopy (vEM) methods along with the complexity and heterogeneity of environmental samples pose great technical challenges. In the present study, we used a workflow based on a specific electron microscopy sample preparation method compatible with both light and vEM imaging in order to target one cell among a complex natural community. This method revealed the 3D subcellular landscape of a photosynthetic dinoflagellate, which we identified as Ensiculifera tyrrhenica, with quantitative characterization of multiple organelles. We show that this cell contains a single convoluted chloroplast and show the arrangement of the flagellar apparatus with its associated photosensitive elements. Moreover, we observed partial chromatin unfolding, potentially associated with transcription activity in these organisms, in which chromosomes are permanently condensed. Together with providing insights in dinoflagellate biology, this proof-of-principle study illustrates an efficient tool for the targeted ultrastructural analysis of environmental microorganisms in heterogeneous mixes.
Keywords: Correlative light and electron microscopy; Dinoflagellate; Environmental sample; Focused ion beam-scanning electron microscopy; Plankton; Volume electron microscopy.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures
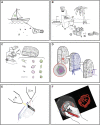

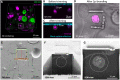
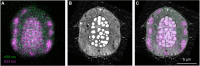



References
-
- Calado, A. J. and Moestrup, Ø. (2002). Ultrastructural study of the type species of Peridiniopsis, Peridiniopsis borgei (Dinophyceae), with special reference to the peduncle and flagellar apparatus. Phycologia 41, 567-584. 10.2216/i0031-8884-41-6-567.1 - DOI
-
- Calado, A. J., Hansen, G. and Moestrup, Ø. (1999). Architecture of the flagellar apparatus and related structures in the type species of peridinium, p. cinctum (dinophyceae). Eur. J. Phycol. 34, 179-191. 10.1080/09670269910001736232 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

