Vascular endothelial growth factor isoforms differentially protect neurons against neurotoxic events associated with Alzheimer's disease
- PMID: 37456522
- PMCID: PMC10349181
- DOI: 10.3389/fnmol.2023.1181626
Vascular endothelial growth factor isoforms differentially protect neurons against neurotoxic events associated with Alzheimer's disease
Abstract
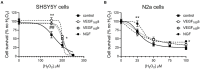
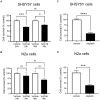
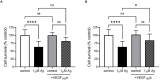
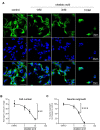
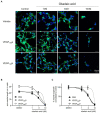
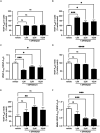
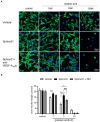
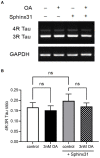
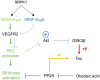
Alzheimer's disease (AD) is the most common cause of dementia, the chronic and progressive deterioration of memory and cognitive abilities. AD can be pathologically characterised by neuritic plaques and neurofibrillary tangles, formed by the aberrant aggregation of β-amyloid and tau proteins, respectively. We tested the hypothesis that VEGF isoforms VEGF-A165a and VEGF-A165b, produced by differential splice site selection in exon 8, could differentially protect neurons from neurotoxicities induced by β-amyloid and tau proteins, and that controlling expression of splicing factor kinase activity could have protective effects on AD-related neurotoxicity in vitro. Using oxidative stress, β-amyloid, and tau hyperphosphorylation models, we investigated the effect of VEGF-A splicing isoforms, previously established to be neurotrophic agents, as well as small molecule kinase inhibitors, which selectively inhibit SRPK1, the major regulator of VEGF splicing. While both VEGF-A165a and VEGF-A165b isoforms were protective against AD-related neurotoxicity, measured by increased metabolic activity and neurite outgrowth, VEGF-A165a was able to enhance neurite outgrowth but VEGF-A165b did not. In contrast, VEGF-A165b was more effective than VEGF-A165a in preventing neurite "dieback" in a tau hyperphosphorylation model. SRPK1 inhibition was found to significantly protect against neurite "dieback" through shifting AS of VEGFA towards the VEGF-A165b isoform. These results indicate that controlling the activities of the two different isoforms could have therapeutic potential in Alzheimer's disease, but their effect may depend on the predominant mechanism of the neurotoxicity-tau or β-amyloid.
Keywords: Alzheimer’s disease; SRPK1; VEGF; amyloid-beta; splicing; tau.
Copyright © 2023 Alalwany, Hawtrey, Morgan, Morris, Donaldson and Bates.
Conflict of interest statement
LD, DB, and JM are founders and stock-holders in Exonate Ltd., a company that is developing SRPK1 inhibitors for clinical use. LD and JM are founders and stockholders in Emenda Therapeutics, a company that is developing splicing factor kinase inhibitors for therapeutic use. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bancher C., Brunner C., Lassmann H., Budka H., Jellinger K., Wiche G., et al. . (1989). Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer's disease. Brain Res. 477, 90–99. PMID: - PubMed
-
- Beazley-Long N., Hua J., Jehle T., Hulse R. P., Dersch R., Lehrling C., et al. . (2013). VEGF-A165b is an endogenous Neuroprotective splice isoform of vascular endothelial growth factor a in vivo and in vitro. Am. J. Pathol. 183, 918–929. doi: 10.1016/j.ajpath.2013.05.031, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

