Probiotics formulation and cancer nanovaccines show synergistic effect in immunotherapy and prevention of colon cancer
- PMID: 37456845
- PMCID: PMC10338235
- DOI: 10.1016/j.isci.2023.107167
Probiotics formulation and cancer nanovaccines show synergistic effect in immunotherapy and prevention of colon cancer
Abstract
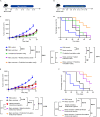
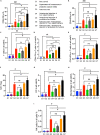
Probiotics play essential roles in immune modulation. Combining probiotics with cancer vaccines potentially can achieve a synergistic effect. To maximize the efficacy of probiotics, proper probiotics formulation is necessary. Herein, Lactobacillus rhamnosus and Bifidobacterium longum are coated with lipid membrane to achieve the goal of losing less activity and bettering colonization in colon. In the subcutaneous transplanted colon cancer mouse model, probiotics formulation showed potent preventive and therapeutic efficacy, and the efficacy could be further improved by combining with cancer nanovaccines. Probiotics formulation can perform as immune adjuvants to enhance the innate immune response or as in-situ cancer vaccines. In the study of preventing chemical-induced orthotopic colon cancer model, probiotics formulation alone efficiently reduced tumor number in colon and the efficacy is improved by combining with cancer nanovaccines. All in all, the studies demonstrated that probiotics formulation can assist to maximize the efficacy of cancer nanovaccines.
Keywords: Biological sciences; Biotechnology; Cancer; Immunology; Microbiology; Nanocomplex.
© 2023 The Author(s).
Conflict of interest statement
M.L. is a shareholder of Suzhou Ersheng Biopharmaceutical Co., Ltd, Suzhou, People’s Republic of China. M.L. has applied a patent related to this study. The remaining authors have reported no financial interests or potential conflicts of interest.
Figures








References
-
- Gao J., Logan K.A., Nesbitt H., Callan B., McKaig T., Taylor M., Love M., McHale A.P., Griffith D.M., Callan J.F. A single microbubble formulation carrying 5-fluorouridine, Irinotecan and oxaliplatin to enable FOLFIRINOX treatment of pancreatic and colon cancer using ultrasound targeted microbubble destruction. J. Contr. Release. 2021;338:358–366. doi: 10.1016/j.jconrel.2021.08.050. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

