Adipose-derived stem cells modulate neuroinflammation and improve functional recovery in chronic constriction injury of the rat sciatic nerve
- PMID: 37457010
- PMCID: PMC10339833
- DOI: 10.3389/fnins.2023.1172740
Adipose-derived stem cells modulate neuroinflammation and improve functional recovery in chronic constriction injury of the rat sciatic nerve
Abstract
Introduction: Compressive neuropathy, a common chronic traumatic injury of peripheral nerves, leads to variable impairment in sensory and motor function. Clinical symptoms persist in a significant portion of patients despite decompression, with muscle atrophy and persistent neuropathic pain affecting 10%-25% of cases. Excessive inflammation and immune cell infiltration in the injured nerve hinder axon regeneration and functional recovery. Although adipose-derived stem cells (ASCs) have demonstrated neural regeneration and immunomodulatory potential, their specific effects on compressive neuropathy are still unclear.
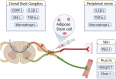
Methods: We conducted modified CCI models on adult male Sprague-Dawley rats to induce irreversible neuropathic pain and muscle atrophy in the sciatic nerve. Intraneural ASC injection and nerve decompression were performed. Behavioral analysis, muscle examination, electrophysiological evaluation, and immunofluorescent examination of the injured nerve and associated DRG were conducted to explore axon regeneration, neuroinflammation, and the modulation of inflammatory gene expression. Transplanted ASCs were tracked to investigate potential beneficial mechanisms on the local nerve and DRG.
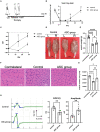
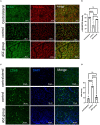
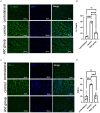
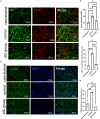
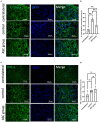
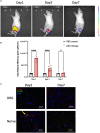
Results: Persistent neuropathic pain was induced by chronic constriction of the rat sciatic nerve. Local ASC treatment has demonstrated robust beneficial outcomes, including the alleviation of mechanical allodynia, improvement of gait, regeneration of muscle fibers, and electrophysiological recovery. In addition, locally transplanted ASCs facilitated axon remyelination, alleviated neuroinflammation, and reduced inflammatory cell infiltration of the injured nerve and associated dorsal root ganglion (DRG). Trafficking of the transplanted ASC preserved viability and phenotype less than 7 days but contributed to robust immunomodulatory regulation of inflammatory gene expression in both the injured nerve and DRG.
Discussion: Locally transplanted ASC on compressed nerve improve sensory and motor recoveries from irreversible chronic constriction injury of rat sciatic nerve via alleviation of both local and remote neuroinflammation, suggesting the promising role of adjuvant ASC therapies for clinical compressive neuropathy.
Keywords: adipose derived stem cells; chronic constriction injury; compressive neuropathy; immunomodulation; neuroinflammation; neuropathic pain; stem cell therapy.
Copyright © 2023 Chen, Wu, Tseng, Lu, Liu, Lin, Lin and Hsueh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

