Osteocyte β3 integrin promotes bone mass accrual and force-induced bone formation in mice
- PMID: 37457310
- PMCID: PMC10338905
- DOI: 10.1016/j.jot.2023.05.001
Osteocyte β3 integrin promotes bone mass accrual and force-induced bone formation in mice
Abstract
Background: Cell culture studies demonstrate the importance of β3 integrin in osteocyte mechanotransduction. However, the in vivo roles of osteocyte β3 integrin in the regulation of bone homeostasis and mechanotransduction are poorly defined.
Materials and methods: To study the in vivo role of osteocyte β3 integrin in bone, we utilized the 10-kb Dmp1 (dentin matrix acidic phosphoprotein 1)-Cre to delete β3 integrin expression in osteocyte in mice. Micro-computerized tomography (μCT), bone histomorphometry and in vitro cell culture experiments were performed to determine the effects of osteocyte β3 integrin loss on bone mass accrual and biomechanical properties. In addition, in vivo tibial loading model was applied to study the possible involvement of osteocyte β3 integrin in the mediation of bone mechanotransduction.
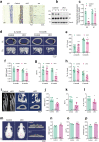
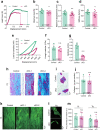
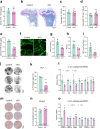
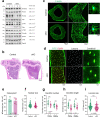
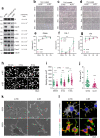
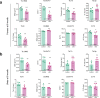
Results: Deletion of β3 integrin in osteocytes resulted in a low bone mass and impaired biomechanical properties in load-bearing long bones in adult mice. The loss of β3 integrin led to abnormal cell morphology with reduced number and length of dentritic processes in osteocytes. Furthermore, osteocyte β3 integrin loss did not impact the osteoclast formation, but significantly reduced the osteoblast-mediated bone formation rate and reduced the osteogenic differentiation of the bone marrow stromal cells in the bone microenvironment. In addition, mechanical loading failed to accelerate the anabolic bone formation in mutant mice.
Conclusions: Our studies demonstrate the essential roles of osteocyte β3 integrin in regulating bone mass and mechanotransduction.
Keywords: Bone homeostasis; Mechanotransduction; Osteocyte; β3 integrin.
© 2023 Published by Elsevier B.V. on behalf of Chinese Speaking Orthopaedic Society.
Conflict of interest statement
A conflict of interest occurs when an individual's objectivity is potentially compromised by a desire for financial gain, prominence, professional advancement or a successful outcome. The Editors of the Journal of Orthopaedic Translation strive to ensure that what is published in the Journal is as balanced, objective and evidence-based as possible. Since it can be difficult to distinguish between an actual conflict of interest and a perceived conflict of interest, the Journal requires authors to disclose all and any potential conflicts of interest.
Figures





Similar articles
-
Osteocyte β1 integrin loss causes low bone mass and impairs bone mechanotransduction in mice.J Orthop Translat. 2022 May 17;34:60-72. doi: 10.1016/j.jot.2022.03.008. eCollection 2022 May. J Orthop Translat. 2022. PMID: 35615639 Free PMC article.
-
Potential role for a specialized β3 integrin-based structure on osteocyte processes in bone mechanosensation.J Orthop Res. 2018 Feb;36(2):642-652. doi: 10.1002/jor.23792. Epub 2017 Nov 28. J Orthop Res. 2018. PMID: 29087614 Free PMC article.
-
Dentin matrix protein 1 gene cis-regulation: use in osteocytes to characterize local responses to mechanical loading in vitro and in vivo.J Biol Chem. 2005 May 27;280(21):20680-90. doi: 10.1074/jbc.M500104200. Epub 2005 Feb 22. J Biol Chem. 2005. PMID: 15728181
-
Integrins in Osteocyte Biology and Mechanotransduction.Curr Osteoporos Rep. 2019 Aug;17(4):195-206. doi: 10.1007/s11914-019-00520-2. Curr Osteoporos Rep. 2019. PMID: 31250372 Review.
-
The Osteocyte as the New Discovery of Therapeutic Options in Rare Bone Diseases.Front Endocrinol (Lausanne). 2020 Jul 8;11:405. doi: 10.3389/fendo.2020.00405. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32733380 Free PMC article. Review.
Cited by
-
Research progress on the regulatory mechanism of integrin-mediated mechanical stress in cells involved in bone metabolism.J Cell Mol Med. 2024 Apr;28(7):e18183. doi: 10.1111/jcmm.18183. J Cell Mol Med. 2024. PMID: 38506078 Free PMC article. Review.
-
Novel homozygous frameshift mutation of ITGB3 in the Glanzmann thrombasthenia patient with abnormal bone metabolism and congenital bone defects.Orphanet J Rare Dis. 2025 Apr 18;20(1):189. doi: 10.1186/s13023-025-03700-9. Orphanet J Rare Dis. 2025. PMID: 40251671 Free PMC article.
-
Osteocytes function as biomechanical signaling hubs bridging mechanical stress sensing and systemic adaptation.Front Physiol. 2025 Jul 15;16:1629273. doi: 10.3389/fphys.2025.1629273. eCollection 2025. Front Physiol. 2025. PMID: 40735673 Free PMC article. Review.
-
Targeting Piezo1 channel to alleviate intervertebral disc degeneration.J Orthop Translat. 2025 Mar 8;51:145-158. doi: 10.1016/j.jot.2025.01.006. eCollection 2025 Mar. J Orthop Translat. 2025. PMID: 40129609 Free PMC article.
-
Advanced Piezoelectric Materials, Devices, and Systems for Orthopedic Medicine.Adv Sci (Weinh). 2025 Jan;12(3):e2410400. doi: 10.1002/advs.202410400. Epub 2024 Dec 12. Adv Sci (Weinh). 2025. PMID: 39665130 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

