Synthesis of N-Acylsulfenamides from Amides and N-Thiosuccinimides
- PMID: 37457378
- PMCID: PMC10348737
- DOI: 10.1055/s-0041-1738430
Synthesis of N-Acylsulfenamides from Amides and N-Thiosuccinimides
Abstract
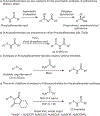
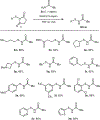
Herein is reported a robust and general method for the preparation of N-acylsulfenamides, important functionalities that have recently been utilized as central inputs for the asymmetric synthesis of high oxidation state sulfur compounds. This straightforward transformation proceeds by reaction of primary amides, carbamates, sulfonamides, sulfinamides, and ureas with stable N-thiosuccinimides or N-thiophthalimides, which in turn are prepared in a single step from commercial thiols. The use of stable N-thiosuccinimide and N-thiophthalimide reactants is desirable because it obviates the use of highly reactive sulfenyl chlorides.
Keywords: amide; asymmetric catalysis; sulfenamide; thiol; thiophthalimide; thiosuccinimide.
Conflict of interest statement
Conflict of Interest The authors declare no conflict of interest.
Figures
References
-
- Ma L; Bai L; Yu Z; Shen Q Chirality 2022, 34, 1191. - PubMed
-
- Behforouz M; Kerwood JE J. Org. Chem 1969, 34, 51.
- Harpp DN; Mullins DF; Steliou K; Triassi IJ Org. Chem 1979, 44, 4196.
- Koval IV; Tarasenko AI; Kremlev MM; Molchanova NR Zh. Org. Khim 1981, 17, 533.
- Miura Y; Shibata Y; Kinoshita M Bull. Chem. Soc. Jpn 1986, 59, 3291.
- Perrio S; Reboul V; Metzner P In Science of Synthesis., Vol. 31a; Ramsden CA, Ed.; Georg Thieme Verlag: Stuttgart, 2007, 1041.
- Ma L-J; Chen S-S; Li G-X; Zhu J; Wang Q-W; Tang Z ACS Catal. 2019, 9, 1525.
Grants and funding
LinkOut - more resources
Full Text Sources