Synthesis and Characterization of Novel Thienothiadiazole-Based D-π-A-π-D Fluorophores as Potential NIR Imaging Agents
- PMID: 37457472
- PMCID: PMC10339328
- DOI: 10.1021/acsomega.3c02602
Synthesis and Characterization of Novel Thienothiadiazole-Based D-π-A-π-D Fluorophores as Potential NIR Imaging Agents
Abstract
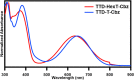
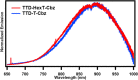
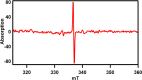
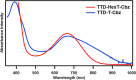
As fluorescence bioimaging has increased in popularity, there have been numerous reports on designing organic fluorophores with desirable properties amenable to perform this task, specifically fluorophores with emission in the near-infrared II (NIR-II) region. One such strategy is to utilize the donor-π-acceptor-π-donor approach (D-π-A-π-D), as this allows for control of the photophysical properties of the resulting fluorophores through modulation of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energy levels. Herein, we illustrate the properties of thienothiadiazole (TTD) as an effective acceptor moiety in the design of NIR emissive fluorophores. TTD is a well-known electron-deficient species, but its use as an acceptor in D-π-A-π-D systems has not been extensively studied. We employed TTD as an acceptor unit in a series of two fluorophores and characterized the photophysical properties through experimental and computational studies. Both fluorophores exhibited emission maxima in the NIR-I that extends into the NIR-II. We also utilized electron paramagnetic resonance (EPR) spectroscopy to rationalize differences in the measured quantum yield values and demonstrated, to our knowledge, the first experimental evidence of radical species on a TTD-based small-molecule fluorophore. Encapsulation of the fluorophores using a surfactant formed polymeric nanoparticles, which were studied by photophysical and morphological techniques. The results of this work illustrate the potential of TTD as an acceptor in the design of NIR-II emissive fluorophores for fluorescence bioimaging applications.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Maulvi F. A.; Lakdawala D. H.; Shaikh A. A.; Desai A. R.; Choksi H. H.; Vaidya R. J.; Ranch K. M.; Koli A. R.; Vyas B. A.; Shah D. O. In Vitro and in Vivo Evaluation of Novel Implantation Technology in Hydrogel Contact Lenses for Controlled Drug Delivery. J. Controlled Release 2016, 226, 47–56. 10.1016/j.jconrel.2016.02.012. - DOI - PubMed
-
- Shen Q.; Wang S.; Yang N.-D.; Zhang C.; Wu Q.; Yu C. Recent Development of Small-Molecule Organic Fluorophores for Multifunctional Bioimaging in the Second near-Infrared Window. J. Lumin. 2020, 225, 11733810.1016/j.jlumin.2020.117338. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

