BL-MOL-AR Project, Preliminary Results about Liquid Biopsy: Molecular Approach Experience and Research Activity in Oncological Settings
- PMID: 37457625
- PMCID: PMC10348843
- DOI: 10.1055/s-0043-1771193
BL-MOL-AR Project, Preliminary Results about Liquid Biopsy: Molecular Approach Experience and Research Activity in Oncological Settings
Abstract
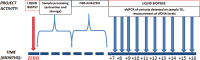
Background Liquid biopsy is mainly used to identify tumor cells in pulmonary neoplasms. It is more often used in research than in clinical practice. The BL-MOL-AR study aims to investigate the efficacy of next-generation sequencing (NGS) and clinical interpretation of the circulating free DNA (cfDNA) levels. This study reports the preliminary results from the first samples analyzed from patients affected by various neoplasms: lung, intestinal, mammary, gastric, biliary, and cutaneous. Methods The Biopsia Liquida-Molecolare-Arezzo study aims to enroll cancer patients affected by various malignancies, including pulmonary, intestinal, advanced urothelial, biliary, breast, cutaneous, and gastric malignancies. Thirty-nine patients were included in this preliminary report. At time zero, a liquid biopsy is executed, and two types of NGS panels are performed, comprising 17 genes in panel 1, which is already used in the routine tissue setting, and 52 genes in panel 2. From the 7th month after enrollment, 10 sequential liquid biopsies are performed up to the 17th month. The variant allele frequency (%) and cfDNA levels (ng/mL) are measured in every plasmatic sample. Results The NGS results obtained by different panels are similar even though the number of mutations is more concordant for lung pathologies. There are no significant differences in the actionability levels of the identified variants. Most of the molecular profiles of liquid biopsies reflect tissue data. Conclusions Preliminary data from this study confirm the need to clarify the limitations and potential of liquid biopsy beyond the lung setting. Overall, parameters related to cfDNA levels and variant allele frequency could provide important indications for prognosis and disease monitoring.
Keywords: NGS; cfDNA; liquid biopsy.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. ( https://creativecommons.org/licenses/by/4.0/ ).
Conflict of interest statement
Conflict of Interest None declared.
Figures





References
-
- Malapelle U, Pisapia P, Addeo A. Liquid biopsy from research to clinical practice: focus on non-small cell lung cancer. Expert Rev Mol Diagn. 2021;21(11):1165–1178. - PubMed
-
- The global challenge of cancer. Nat Cancer. 2020;1(01):1–2. - PubMed
-
- Gao W, Huang T, Yuan H. Highly sensitive detection and mutational analysis of lung cancer circulating tumor cells using integrated combined immunomagnetic beads with a droplet digital PCR chip. Talanta. 2018;185:229–236. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
