Effectiveness and safety of anti-IL-5/Rα biologics in eosinophilic granulomatosis with polyangiitis: a two-year multicenter observational study
- PMID: 37457743
- PMCID: PMC10349177
- DOI: 10.3389/fimmu.2023.1204444
Effectiveness and safety of anti-IL-5/Rα biologics in eosinophilic granulomatosis with polyangiitis: a two-year multicenter observational study
Abstract
Background: Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare vasculitis characterized by asthma, systemic manifestations, and blood and tissue eosinophilia.
Objective: To assess the effectiveness and safety of mepolizumab (anti-IL-5) and benralizumab (anti-IL-5Rα) in EGPA for 24 months.
Methods: We conducted a multicenter observational study, including patients with EGPA treated with anti-IL-5/Rα biologics in 9 Italian specialized facilities. Systemic disease activity, remission and relapse rate were evaluated from 3 to 24 months after treatment initiation. Respiratory outcomes, hematological parameters, corticosteroid (OCS) and immunosuppressants consumption were also assessed.
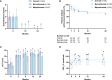
Results: 49 patients with relapsing-refractory EGPA were included [26 (53.1%) benralizumab 30mg, 20 (40.8%) mepolizumab 100mg, 3 (6.1%) mepolizumab 300mg]. Overall, 38.8% and 57.1% achieved remission after 12 and 24 months, respectively (69.2% benralizumab and 43.5% mepolizumab). Lower OCS intake and higher blood eosinophil count at baseline were associated with remission at 24 months. Both biologics exerted beneficial effects on severe asthma outcomes. Indeed, 61.2% (61.5% benralizumab and 60.8% mepolizumab) remained exacerbation-free during treatment. Lung function parameters showed improvements in the overall cohort (all p<0.05), but began to decline from month 12, especially with mepolizumab. Marked reduction in blood eosinophils was registered with mepolizumab (p<0.0001), while benralizumab depleted both eosinophils (p<0.0001) and basophils (p<0.0001). In general, 69.6% (76% benralizumab and 61.9% mepolizumab) of OCS-dependent patients lowered their daily dose by 75%, while 28.3% discontinued these drugs. Immunosuppressants were suspended in 88.2% of cases. Adverse events were reported in 8.2% of patients.
Conclusions: These real-world data suggest that anti-IL-5/Rα biologics are effective and safe in the long-term as add-on treatments for patients with EGPA.
Keywords: Churg-Strauss; EGPA; IL-5; benralizumab; eosinophilic granulomatosis with polyangiitis; mepolizumab; oral corticosteroid; remission.
Copyright © 2023 Nolasco, Portacci, Campisi, Buonamico, Pelaia, Benfante, Triggiani, Spadaro, Caiaffa, Scioscia, Detoraki, Valenti, Papia, Tomasello, Crimi, Scichilone, Pelaia, Carpagnano and Crimi.
Conflict of interest statement
AP has received speaker fees from AstraZeneca, GlaxoSmithKline, Chiesi and Sanofi. AB has received speaker fees from AstraZeneca, GlaxoSmithKline, and Sanofi. MT has received speaker and consultancy fees from Novartis, AstraZeneca and GlaxoSmithKline. GSc has received speaker fees from AstraZeneca, GlaxoSmithKline, and Sanofi. AD has received speaker fees from Sanofi, AstraZeneca, GlaxoSmithKline, Novartis, Lofarma. GV has received speaker fees from GlaxoSmithKline, and Sanofi. NC has received speaker and lecturer fees from AstraZeneca, GlaxoSmithKline and Sanofi. NS has received speaker fees from AstraZeneca, GlaxoSmithKline, Chiesi and Sanofi. GP has received speaker and lecturer fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Guidotti, Menarini, Novartis and Sanofi. GC has received grants from AstraZeneca, GlaxoSmithKline, Chiesi, Sanofi and Grifols; has received speaker and lecturer fees from AstraZeneca, GlaxoSmithKline, and Sanofi; has received supports for attending meetings from AstraZeneca, Menarini and Chiesi. CC has received speaker fees from ResMed, Fisher&Paykel, Sanofi, AstraZeneca, GlaxoSmithKline and Menarini. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor EH declared a past co-authorship with the authors SN, RC, CP, AB, MT, GS, MC, AD, NC, NS, GP, GC and CC.
Figures




References
-
- Grayson PC, Ponte C, Suppiah R, Robson JC, Craven A, Judge A, et al. . American College of Rheumatology/European alliance of associations for rheumatology classification criteria for eosinophilic granulomatosis with polyangiitis. Arthritis Rheumatol (2022) 74(3):386–92. doi: 10.1002/art.41982 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

