EPD1504: a novel μ-opioid receptor partial agonist attenuates obsessive-compulsive disorder (OCD)-like behaviors
- PMID: 37457777
- PMCID: PMC10349350
- DOI: 10.3389/fpsyt.2023.1170541
EPD1504: a novel μ-opioid receptor partial agonist attenuates obsessive-compulsive disorder (OCD)-like behaviors
Abstract
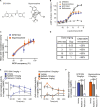
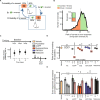
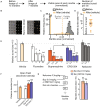
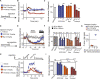
Low doses of μ-opioid receptor (MOR) agonists rapidly ameliorate symptoms in treatment-resistant obsessive-compulsive disorder (OCD) patients (10-50% of OCD patients). However, the utility of MOR agonists is limited by their safety liabilities. We developed a novel MOR partial agonist (EPD1540) that has an improved respiratory safety profile when compared to buprenorphine. Buprenorphine is a MOR partial agonist primarily used in the treatment of opiate-use disorder, which in investigator-led trials, has been shown to rapidly ameliorate symptoms in treatment-resistant OCD patients. In this study, we show that doses of EPD1504 and buprenorphine that occupy small fractions of MORs in the CNS (approximately 20%) are as effective as fluoxetine at ameliorating OCD-like behaviors in two different rat models (an operant probabilistic reversal task and marble burying). Importantly, effective doses of EPD1504 did not impair either locomotor activity, or respiration under normoxic or hypercapnic conditions. Additionally, EPD1504 had effects comparable to buprenorphine in the conditioned place preference assay. These results indicate that EPD1504 may provide a safer alternative to buprenorphine for the treatment of OCD patients.
Keywords: buprenorphine (BN); marble burying (MB); obsessive–compulsive disorder (OCD); opioid; partial agonist; probabilistic reversal task; treatment resistant OCD.
Copyright © 2023 Youngblood, Medina, Gehlert and Schwartz.
Conflict of interest statement
BY, DG, and NS are employees of and stockholders in Epiodyne, Inc. JM is an Epiodyne stockholder, and a stockholder and employee of R2M.
Figures
Similar articles
-
Behavioral Effects of a Novel Benzofuranyl-Piperazine Serotonin-2C Receptor Agonist Suggest a Potential Therapeutic Application in the Treatment of Obsessive-Compulsive Disorder.Front Psychiatry. 2017 May 22;8:89. doi: 10.3389/fpsyt.2017.00089. eCollection 2017. Front Psychiatry. 2017. PMID: 28588509 Free PMC article.
-
Buprenorphine augmentation improved symptoms of OCD, compared to placebo - Results from a randomized, double-blind and placebo-controlled clinical trial.J Psychiatr Res. 2017 Nov;94:23-28. doi: 10.1016/j.jpsychires.2017.06.004. Epub 2017 Jun 13. J Psychiatr Res. 2017. PMID: 28647677 Clinical Trial.
-
Neurosteroids modulate compulsive and persistent behavior in rodents: implications for obsessive-compulsive disorder.Prog Neuropsychopharmacol Biol Psychiatry. 2009 Oct 1;33(7):1161-6. doi: 10.1016/j.pnpbp.2009.06.013. Epub 2009 Jun 21. Prog Neuropsychopharmacol Biol Psychiatry. 2009. PMID: 19549549
-
Animal models of obsessive-compulsive disorder: rationale to understanding psychobiology and pharmacology.Psychiatr Clin North Am. 2006 Jun;29(2):371-90. doi: 10.1016/j.psc.2006.02.007. Psychiatr Clin North Am. 2006. PMID: 16650714 Review.
-
Hypochondriasis and its relationship to obsessive-compulsive disorder.Psychiatr Clin North Am. 2000 Sep;23(3):605-16. doi: 10.1016/s0193-953x(05)70183-0. Psychiatr Clin North Am. 2000. PMID: 10986730 Review.
References
-
- Ecker AH, Stanley MA, Smith TL, Teng EJ, Fletcher TL, Van Kirk N, et al. . Co-occurrence of obsessive–compulsive disorder and substance use disorders among U.S. veterans: prevalence and mental health utilization. J Cogn Psychother. (2019) 33:23–32. doi: 10.1891/0889-8391.33.1.23, PMID: - DOI - PubMed
-
- Ahmadpanah M, Reihani A, Ghaleiha A, Soltanian A, Haghighi M, Jahangard L, et al. . Buprenorphine augmentation improved symptoms of OCD, compared to placebo - results from a randomized, double-blind, and placebo-controlled clinical trial. J Psychiatr Res. (2017) 94:23–8. doi: 10.1016/j.jpsychires.2017.06.004, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

