Inflammation in the pathogenesis of depression: a disorder of neuroimmune origin
- PMID: 37457896
- PMCID: PMC10345431
- DOI: 10.1042/NS20220054
Inflammation in the pathogenesis of depression: a disorder of neuroimmune origin
Abstract
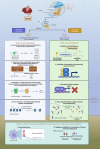
There are several hypotheses concerning the underlying pathophysiological mechanisms of major depression, which centre largely around adaptive changes in neuronal transmission and plasticity, neurogenesis, and circuit and regional connectivity. The immune and endocrine systems are commonly implicated in driving these changes. An intricate interaction of stress hormones, innate immune cells and the actions of soluble mediators of immunity within the nervous system is described as being associated with the symptoms of depression. Bridging endocrine and immune processes to neurotransmission and signalling within key cortical and limbic brain circuits are critical to understanding depression as a disorder of neuroimmune origins. Emergent areas of research include a growing recognition of the adaptive immune system, advances in neuroimaging techniques and mechanistic insights gained from transgenic animals. Elucidation of glial-neuronal interactions is providing additional avenues into promising areas of research, the development of clinically relevant disease models and the discovery of novel therapies. This narrative review focuses on molecular and cellular mechanisms that are influenced by inflammation and stress. The aim of this review is to provide an overview of our current understanding of depression as a disorder of neuroimmune origin, focusing on neuroendocrine and neuroimmune dysregulation in depression pathophysiology. Advances in current understanding lie in pursuit of relevant biomarkers, as the potential of biomarker signatures to improve clinical outcomes is yet to be fully realised. Further investigations to expand biomarker panels including integration with neuroimaging, utilising individual symptoms to stratify patients into more homogenous subpopulations and targeting the immune system for new treatment approaches will help to address current unmet clinical need.
Keywords: Depression; Inflammation; Neuroimmunology.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



References
-
- McGuinness B. and Harkin A. (2015) Rodent models of stress-induced depression: the link between stress and immune system related changes. Immunology and Psychiatry, pp. 33–62, Springer, Cham: 10.1007/978-3-319-13602-8_3 - DOI
Publication types
LinkOut - more resources
Full Text Sources

