Simufilam suppresses overactive mTOR and restores its sensitivity to insulin in Alzheimer's disease patient lymphocytes
- PMID: 37457922
- PMCID: PMC10339288
- DOI: 10.3389/fragi.2023.1175601
Simufilam suppresses overactive mTOR and restores its sensitivity to insulin in Alzheimer's disease patient lymphocytes
Expression of concern in
-
Expression of concern: Simufilam suppresses overactive mTOR and restores its sensitivity to insulin in Alzheimer's disease patient lymphocytes.Front Aging. 2024 Aug 22;5:1483030. doi: 10.3389/fragi.2024.1483030. eCollection 2024. Front Aging. 2024. PMID: 39239266 Free PMC article. No abstract available.
Abstract
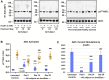
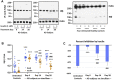
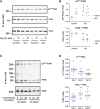
Introduction: Implicated in both aging and Alzheimer's disease (AD), mammalian target of rapamycin (mTOR) is overactive in AD brain and lymphocytes. Stimulated by growth factors such as insulin, mTOR monitors cell health and nutrient needs. A small molecule oral drug candidate for AD, simufilam targets an altered conformation of the scaffolding protein filamin A (FLNA) found in AD brain and lymphocytes that induces aberrant FLNA interactions leading to AD neuropathology. Simufilam restores FLNA's normal shape to disrupt its AD-associated protein interactions. Methods: We measured mTOR and its response to insulin in lymphocytes of AD patients before and after oral simufilam compared to healthy control lymphocytes. Results: mTOR was overactive and its response to insulin reduced in lymphocytes from AD versus healthy control subjects, illustrating another aspect of insulin resistance in AD. After oral simufilam, lymphocytes showed normalized basal mTOR activity and improved insulin-evoked mTOR activation in mTOR complex 1, complex 2, and upstream and downstream signaling components (Akt, p70S6K and phosphorylated Rictor). Suggesting mechanism, we showed that FLNA interacts with the insulin receptor until dissociation by insulin, but this linkage was elevated and its dissociation impaired in AD lymphocytes. Simufilam improved the insulin-mediated dissociation. Additionally, FLNA's interaction with Phosphatase and Tensin Homolog deleted on Chromosome 10 (PTEN), a negative regulator of mTOR, was reduced in AD lymphocytes and improved by simufilam. Discussion: Reducing mTOR's basal overactivity and its resistance to insulin represents another mechanism of simufilam to counteract aging and AD pathology. Simufilam is currently in Phase 3 clinical trials for AD dementia.
Keywords: Alzheimer’s disease; PTEN; aging; filamin A; insulin resistance; mTOR.
Copyright © 2023 Wang, Pei, Lee, Nikolov, Doehner, Puente, Friedmann and Burns.
Conflict of interest statement
This basic research was supported in part by supplies and funding from Cassava Sciences, Inc. LB is an employee and shareholder of Cassava Sciences, as was the late NF. H-YW is an employee of City University of New York School of Medicine, is a long-time consultant and scientific advisor to Cassava Sciences, owns an insignificant financial interest (less than ½ of 1%) in the equity of Cassava Sciences, and has consulted to various pharmaceutical companies over the past 20 years. LB and H-YW are inventors on simufilam patents. None of the authors are due patent royalties. This basic research was conducted in non-GCP compliant facilities. ZP and K-CL are employees of City University of New York School of Medicine. BN, TD, and JP are investigators in the clinical trial protocols. Author BN is employed by IMIC, Inc. Authors TD and JP are employed by Cognitive Clinical Trials.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

