Humoral immune responses to inactivated COVID-19 vaccine up to 1 year in children with chronic hepatitis B infection
- PMID: 37457966
- PMCID: PMC10339386
- DOI: 10.3389/fcimb.2023.1201101
Humoral immune responses to inactivated COVID-19 vaccine up to 1 year in children with chronic hepatitis B infection
Abstract
Background: Inactivated SARS-CoV-2 vaccination has recently been approved for children aged 3-17 years in China. However, data on long-term humoral responses to inactivated vaccines in children with chronic hepatitis B (CHB) are still limited.
Methods: In this prospective observational study, CHB children after primary inactivated SARS-CoV-2 vaccines were recruited consecutively and followed up for 1 year. CHB adults from another cohort study (NCT05007665) were used as a control. The receptor-binding domain IgG antibody (anti-RBD-IgG), neutralizing antibody (NAb), neutralization against Omicron (BA2.12.1, BA.4 and BA.5), and memory B -cell (MBC) responses were evaluated.
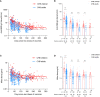
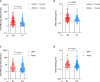
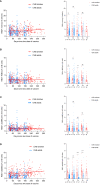
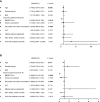
Results: Overall, 115 CHB children and 351 CHB adults were included in this analysis. The antibody titers decreased over the first ~180 days and then plateaued up to 1 year in CHB children. However, lower and faster declines in antibody responses were observed in CHB adults. Interestingly, the seroprevalence of antibodies was still high after over 8 months in CHB children (anti-RBD-IgG [90%] and NAbs [83%]). However, neutralization against Omicron subvariants was significantly reduced in CHB children (-3.68-fold to -8.60-fold). Notably, neutralization against the BA.5 subvariant was obviously diminished in CHB children compared with adults. Moreover, CHB children had similar RBD-specific MBCs but higher RBD-specific atypical MBCs compared with adults.
Conclusion: Inactivated vaccination could elicit more robust and durable antibody responses to the wild-type SARS-CoV-2 strain in CHB children than in CHB adults but showed inferior responses to Omicron subvariants (especially to the BA.5 strain). Hence, new Omicron-related or all-in-one vaccines are needed immediately for CHB children.
Keywords: COVID-19; adolescent; antibody response; chronic hepatitis B; inactivated vaccine; memory B cells; omicron.
Copyright © 2023 Zhou, Chen, He, Peng, Chang, Tan, Li, Cai, Hu, Chen, Peng, Xu and Ren.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Humoral responses after primary and booster SARS-CoV-2 inactivated vaccination in patients with chronic hepatitis B virus infection: A longitudinal observational study.J Med Virol. 2023 Apr;95(4):e28695. doi: 10.1002/jmv.28695. J Med Virol. 2023. PMID: 36946505
-
Safety and immunogenicity of inactivated COVID-19 vaccine in patients with metabolic syndrome: A cross-sectional observational study.Front Public Health. 2022 Dec 23;10:1067342. doi: 10.3389/fpubh.2022.1067342. eCollection 2022. Front Public Health. 2022. PMID: 36620297 Free PMC article.
-
Stronger and durable SARS-CoV-2 immune response to mRNA vaccines in 5-11 years old children with prior COVID-19.Vaccine. 2024 Jan 12;42(2):263-270. doi: 10.1016/j.vaccine.2023.12.006. Epub 2023 Dec 8. Vaccine. 2024. PMID: 38071105
-
Trends of humoral immune responses to heterologous antigenic exposure due to vaccination & omicron SARS-CoV-2 infection: Implications for boosting.Indian J Med Res. 2023 Jun;157(6):509-518. doi: 10.4103/ijmr.ijmr_2521_22. Indian J Med Res. 2023. PMID: 37322634 Free PMC article.
-
Inactivated SARS-CoV-2 booster vaccine enhanced immune responses in patients with chronic liver diseases.Virol Sin. 2023 Oct;38(5):723-734. doi: 10.1016/j.virs.2023.07.005. Epub 2023 Jul 22. Virol Sin. 2023. PMID: 37487943 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

