Parkinson's Progression Markers Initiative: A Milestone-Based Strategy to Monitor Parkinson's Disease Progression
- PMID: 37458046
- PMCID: PMC10578214
- DOI: 10.3233/JPD-223433
Parkinson's Progression Markers Initiative: A Milestone-Based Strategy to Monitor Parkinson's Disease Progression
Abstract
Background: Identifying a meaningful progression metric for Parkinson's disease (PD) that reflects heterogeneity remains a challenge.
Objective: To assess the frequency and baseline predictors of progression to clinically relevant motor and non-motor PD milestones.
Methods: Using data from the Parkinson's Progression Markers Initiative (PPMI) de novo PD cohort, we monitored 25 milestones across six domains ("walking and balance"; "motor complications"; "cognition"; "autonomic dysfunction"; "functional dependence"; "activities of daily living"). Milestones were intended to be severe enough to reflect meaningful disability. We assessed the proportion of participants reaching any milestone; evaluated which occurred most frequently; and conducted a time-to-first-event analysis exploring whether baseline characteristics were associated with progression.
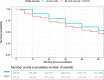
Results: Half of participants reached at least one milestone within five years. Milestones within the cognitive, functional dependence, and autonomic dysfunction domains were reached most often. Among participants who reached a milestone at an annual follow-up visit and remained active in the study, 82% continued to meet criteria for any milestone at one or more subsequent annual visits and 55% did so at the next annual visit. In multivariable analysis, baseline features predicting faster time to reaching a milestone included age (p < 0.0001), greater MDS-UPDRS total scores (p < 0.0001), higher GDS-15 depression scores (p = 0.0341), lower dopamine transporter binding (p = 0.0043), and lower CSF total α-synuclein levels (p = 0.0030). Symptomatic treatment was not significantly associated with reaching a milestone (p = 0.1639).
Conclusion: Clinically relevant milestones occur frequently, even in early PD. Milestones were significantly associated with baseline clinical and biological markers, but not with symptomatic treatment. Further studies are necessary to validate these results, further assess the stability of milestones, and explore translating them into an outcome measure suitable for observational and therapeutic studies.
Keywords: Parkinson’s disease; clinical trials as topic; disease progression; outcome measures.
Conflict of interest statement
The authors have no conflict of interest to report.
MCB receives funding from MJFF.
AS has been a consultant to the following companies in the past year: Biogen, Merck, Denali, Wave Life Sciences and Prilenia Therapeutics. He has received grant funding from the Michael J. Fox Foundation for Parkinson’s Research (MJFF) and NINDS.
TS has served as a consultant for Acadia, Blue Rock Therapeutics, Caraway Therapeutics, Critical Path for Parkinson’s Consortium (CPP), Denali, General Electric (GE), Neuroderm, Sanofi, Sinopia, Sunovion, Roche, Takeda, MJFF, Vanqua Bio and Voyager. She served on the ad board for Acadia, Denali, General Electric (GE), Sunovion, Roche. She has served as a member of the scientific advisory board of Caraway Therapeutics, Neuroderm, Sanofi and UCB. She has received research funding from Biogen, Roche, Neuroderm, Sanofi, Sun Pharma, Amneal, Prevail, UCB, NINDS, MJFF, Parkinson’s Foundation.
EB, SHC, and CCG report no disclosures.
LMC receives research support from MJFF, UPMC Competitive Medical Research Fund, and University of Pittsburgh, is study site investigator for a study sponsored by Biogen, receives research support from NIH, receives consulting fees from Grey Matter Technologies, receives royalties from Elsevier (for authorship), and receives royalties from Wolters Kluwel (for authorship).
BM has received honoraria for consultancy from Roche, Biogen, AbbVie, Servier, 4D Pharma PLC and Amprion. She is a member of the executive steering committee of the Parkinson’s Progression Markers Initiative and PI of the Systemic Synuclein Sampling Study of the Michael J. Fox Foundation for Parkinson’s Research and has received research funding from the Deutsche Forschungsgemeinschaft (DFG), EU (Horizon2020), Parkinson Fonds Deutschland, Deutsche Parkinson Vereinigung, Parkinson’s Foundation, Hilde-Ulrichs-Stiftung für Parkinsonforschung, and MJFF.
TF receives funding from NIH and MJFF.
DG receives research funding from NIH, MJFF, Eli Lilly and Esai. He is a paid Editor for Alzheimer’s Research and Therapy. He is a consultant for Biogen, Roche, GE Healthcare, Fujirebio, Amprion and Generian and serves on a DSMB for Cognition Therapeutics.
KMe receives research funding from MJFF. She is a paid advisor to MJFF and consults for/is on the scientific advisory board for Caraway Therapeutics, Nitrome Biosciences, Nura Bio, Retromer Therapeutics, Sinopia Biosciences, Vanqua Bio and private equity investors.
VA, SJH, and ANO are employees of MJFF.
KLP receives funding from MJFF, NIH, Lewy Body Dementia Association, and Alzheimer’s Drug Discovery Foundation, and receives consulting fees from CuraSen.
CMT is an employee of the University of California – San Francisco and the San Francisco Veterans Affairs Medical Center. She receives grants from the Michael J. Fox Foundation, the Parkinson’s Foundation, the Department of Defense, Roche/Genentech, Biogen Idec, Gateway LLC and NIH; compensation for serving on Data Monitoring Committees from the Parkinson Study Group Partners and Cadent; personal fees for consulting from Adamas Therapeutics, Biogen Idec, CNS Ratings, Australia Parkinson’s Mission, Shake It Up Foundation Australia, Lundbeck Pharmaceuticals and Jazz Pharmaceuticals.
DW has received research funding or support from MJFF, NIH (NINDS), Novartis Pharmaceuticals, Department of Veterans Affairs, Avid Radiopharmaceuticals, Alzheimer’s Disease Cooperative Study, and the International Parkinson and Movement Disorder Society; honoraria for consultancy from Acadia, Biogen, Biotie (Acorda), Bracket, Clintrex LLC, Eisai Inc., Eli Lilly, Lundbeck, Roche, Takeda, UCB, and the CHDI Foundation; license fee payments from the University of Pennsylvania for the QUIP and QUIP-RS; royalties from Wolters Kluweland; and fees for legal consultation for lawsuits related to medication prescribing in patients with Parkinson’s disease.
KK serves as a consultant for Clintrex Research Corp, Blackfynn LLC, Hoover Brown LLC, Roche, Janssen, Lilly, Safe Therapeutics LLP, and Orbimed; and has grant/research support from NIH (NINDS, NCATS) and MJFF.
KMa is a consultant for MJFF, GE Healthcare, Biogen, Roche, Neuropore, OrbiMed, Proclara, Prevail, UCB, Sanofi, Lysosomal Therapetic, Inc, Neuron23, Denali, Takeda, W81XWH-06-1-0678 Establishing an ‘at risk’ cohort for Parkinson Disease Neuroprevention using olfactory testing and DAT imaging, DOD, Investigator 10/1/06 – 09/30/19; Parkinson’s Progression Markers Initiative (PPMI), MJFF, Principal Investigator; DAT imaging in LRRK2 family members, MJFF, Principal Investigator 1/15/10 – 1/14/23. Ownership in Invicro, LLC
CSC receives funding from NINDS, NHLBI, and MJFF.
Figures
References
-
- (1993) Effects of tocopherol and deprenyl on the progression of disability in early Parkinson’s disease. N Engl J Med 328, 176–183. - PubMed
-
- de Lau LM, Schipper CM, Hofman A, Koudstaal PJ, Breteler MM (2005) Prognosis of Parkinson disease: Risk of dementia and mortality: The Rotterdam Study. Arch Neurol 62, 1265–1269. - PubMed
-
- Levy G, Tang MX, Louis ED, Cote LJ, Alfaro B, Mejia H, Stern Y, Marder K (2002) The association of incident dementia with mortality in PD. Neurology 59, 1708–1713. - PubMed
-
- Merola A, Coon EA (2020) Dysautonomia in early Parkinson disease: A window into the determinants of functional disability and an opportunity for early intervention. Clin Auton Res 30, 191–192. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

