Children's Oncology Group's 2023 blueprint for research: Neuroblastoma
- PMID: 37458162
- PMCID: PMC10587593
- DOI: 10.1002/pbc.30572
Children's Oncology Group's 2023 blueprint for research: Neuroblastoma
Abstract
Neuroblastoma is the most common extra-cranial solid tumor in children and is known for its clinical heterogeneity. A greater understanding of the biology of this disease has led to both improved risk stratification and new approaches to therapy. Outcomes for children with low and intermediate risk disease are excellent overall, and efforts to decrease therapy for such patients have been largely successful. Although survival has improved over time for patients with high-risk disease and treatments evaluated in the relapse setting are now being moved into earlier phases of treatment, much work remains to improve survival and decrease therapy-related toxicities. Studies of highly annotated biobanked samples continue to lead to important insights regarding neuroblastoma biology. Such studies, along with correlative biology studies incorporated into therapeutic trials, are expected to continue to provide insights that lead to new and more effective therapies. A focus on translational science is accompanied by an emphasis on new agent development, optimized risk stratification, and international collaboration to address questions relevant to molecularly defined subsets of patients. In addition, the COG Neuroblastoma Committee is committed to addressing the patient/family experience, mitigating late effects of therapy, and studying social determinants of health in patients with neuroblastoma.
Keywords: ALL neuroblastoma; neuroblastoma; neuroblastoma biology.
© 2023 Wiley Periodicals LLC.
Figures
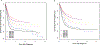

Similar articles
-
Children's Oncology Group's 2023 blueprint for research: Germ cell tumors.Pediatr Blood Cancer. 2023 Sep;70 Suppl 6(Suppl 6):e30562. doi: 10.1002/pbc.30562. Epub 2023 Jul 14. Pediatr Blood Cancer. 2023. PMID: 37449938 Free PMC article.
-
Historical time to disease progression and progression-free survival in patients with recurrent/refractory neuroblastoma treated in the modern era on Children's Oncology Group early-phase trials.Cancer. 2017 Dec 15;123(24):4914-4923. doi: 10.1002/cncr.30934. Epub 2017 Sep 8. Cancer. 2017. PMID: 28885700 Free PMC article. Review.
-
Children's Oncology Group's 2023 blueprint for research: Diversity and health disparities.Pediatr Blood Cancer. 2023 Sep;70 Suppl 6(Suppl 6):e30592. doi: 10.1002/pbc.30592. Epub 2023 Jul 28. Pediatr Blood Cancer. 2023. PMID: 37501542 Free PMC article.
-
Survival of Patients With Neuroblastoma After Assignment to Reduced Therapy Because of the 12- to 18-Month Change in Age Cutoff in Children's Oncology Group Risk Stratification.J Clin Oncol. 2023 Jun 10;41(17):3149-3159. doi: 10.1200/JCO.22.01946. Epub 2023 Apr 25. J Clin Oncol. 2023. PMID: 37098238 Free PMC article.
-
Children's Oncology Group's 2013 blueprint for research: neuroblastoma.Pediatr Blood Cancer. 2013 Jun;60(6):985-93. doi: 10.1002/pbc.24433. Epub 2012 Dec 19. Pediatr Blood Cancer. 2013. PMID: 23255319 Review.
Cited by
-
Targeting Pathways in Neuroblastoma: Advances in Treatment Strategies and Clinical Outcomes.Int J Mol Sci. 2025 May 15;26(10):4722. doi: 10.3390/ijms26104722. Int J Mol Sci. 2025. PMID: 40429864 Free PMC article. Review.
-
The triad in current neuroblastoma challenges: Targeting antigens, enhancing effective cytotoxicity and accurate 3D in vitro modelling.Transl Oncol. 2025 Jan;51:102176. doi: 10.1016/j.tranon.2024.102176. Epub 2024 Nov 2. Transl Oncol. 2025. PMID: 39489087 Free PMC article. Review.
-
Integrated multi-omics characterization of neuroblastoma with bone or bone marrow metastasis.Genes Dis. 2025 Jan 3;12(3):101511. doi: 10.1016/j.gendis.2024.101511. eCollection 2025 May. Genes Dis. 2025. PMID: 40070366 Free PMC article.
-
Interplay Between the Epigenome, the Microenvironment, and the Immune System in Neuroblastoma.Cancers (Basel). 2025 May 29;17(11):1812. doi: 10.3390/cancers17111812. Cancers (Basel). 2025. PMID: 40507292 Free PMC article. Review.
-
Prediction of Composite Clinical Outcomes for Childhood Neuroblastoma Using Multi-Omics Data and Machine Learning.Int J Mol Sci. 2024 Dec 27;26(1):136. doi: 10.3390/ijms26010136. Int J Mol Sci. 2024. PMID: 39795994 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

