Zika virus infection histories in brain development
- PMID: 37458166
- PMCID: PMC10387348
- DOI: 10.1242/dmm.050005
Zika virus infection histories in brain development
Abstract
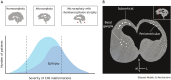
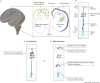
An outbreak of births of microcephalic patients in Brazil motivated multiple studies on this incident. The data left no doubt that infection by Zika virus (ZIKV) was the cause, and that this virus promotes reduction in neuron numbers and neuronal death. Analysis of patients' characteristics revealed additional aspects of the pathology alongside the decrease in neuronal number. Here, we review the data from human, molecular, cell and animal model studies attempting to build the natural history of ZIKV in the embryonic central nervous system (CNS). We discuss how identifying the timing of infection and the pathways through which ZIKV may infect and spread through the CNS can help explain the diversity of phenotypes found in congenital ZIKV syndrome (CZVS). We suggest that intraneuronal viral transport is the primary mechanism of ZIKV spread in the embryonic brain and is responsible for most cases of CZVS. According to this hypothesis, the viral transport through the blood-brain barrier and cerebrospinal fluid is responsible for more severe pathologies in which ZIKV-induced malformations occur along the entire anteroposterior CNS axis.
Keywords: Blood–brain barrier; Brainstem; Calcification; Cerebellum; Neuronal migration; Susceptibility window.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

References
-
- Acosta-Reyes, J., Navarro, E., Herrera, M. J., Goenaga, E., Ospina, M. L., Parra, E., Mercado, M., Chaparro, P., Beltran, M., Gunturiz, M. L.et al. ( 2017). Severe neurologic disorders in 2 fetuses with Zika virus infection, Colombia. Emerg. Infect. Dis. 23, 982- 984. 10.3201/eid2306.161702 - DOI - PMC - PubMed
-
- Aguiar, R. S., Pohl, F., Morais, G. L., Nogueira, F. C. S., Carvalho, J. B., Guida, L., Arge, L. W. P., Melo, A., Moreira, M. E. L., Cunha, D. P.et al. ( 2020). Molecular alterations in the extracellular matrix in the brains of newborns with congenital Zika syndrome. Sci. Signal 13, eaay6736. 10.1126/scisignal.aay6736 - DOI - PubMed
-
- Anderson, D., Neri, J. I. C. F., Souza, C. R. M., Valverde, J. G., De Araújo, J. M. G., Nascimento, M. D. S. B., Branco, R. C. C., Arrais, N. M. R., Lassmann, T., Blackwell, J. M.et al. ( 2021). Zika virus changes methylation of genes involved in immune response and neural development in brazilian babies born with congenital microcephaly. J. Infect. Dis. 223, 435- 440. 10.1093/infdis/jiaa383 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

