Trial of Solanezumab in Preclinical Alzheimer's Disease
- PMID: 37458272
- PMCID: PMC10559996
- DOI: 10.1056/NEJMoa2305032
Trial of Solanezumab in Preclinical Alzheimer's Disease
Abstract
Background: Trials of monoclonal antibodies that target various forms of amyloid at different stages of Alzheimer's disease have had mixed results.
Methods: We tested solanezumab, which targets monomeric amyloid, in a phase 3 trial involving persons with preclinical Alzheimer's disease. Persons 65 to 85 years of age with a global Clinical Dementia Rating score of 0 (range, 0 to 3, with 0 indicating no cognitive impairment and 3 severe dementia), a score on the Mini-Mental State Examination of 25 or more (range, 0 to 30, with lower scores indicating poorer cognition), and elevated brain amyloid levels on 18F-florbetapir positron-emission tomography (PET) were enrolled. Participants were randomly assigned in a 1:1 ratio to receive solanezumab at a dose of up to 1600 mg intravenously every 4 weeks or placebo. The primary end point was the change in the Preclinical Alzheimer Cognitive Composite (PACC) score (calculated as the sum of four z scores, with higher scores indicating better cognitive performance) over a period of 240 weeks.
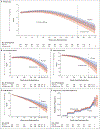
Results: A total of 1169 persons underwent randomization: 578 were assigned to the solanezumab group and 591 to the placebo group. The mean age of the participants was 72 years, approximately 60% were women, and 75% had a family history of dementia. At 240 weeks, the mean change in PACC score was -1.43 in the solanezumab group and -1.13 in the placebo group (difference, -0.30; 95% confidence interval, -0.82 to 0.22; P = 0.26). Amyloid levels on brain PET increased by a mean of 11.6 centiloids in the solanezumab group and 19.3 centiloids in the placebo group. Amyloid-related imaging abnormalities (ARIA) with edema occurred in less than 1% of the participants in each group. ARIA with microhemorrhage or hemosiderosis occurred in 29.2% of the participants in the solanezumab group and 32.8% of those in the placebo group.
Conclusions: Solanezumab, which targets monomeric amyloid in persons with elevated brain amyloid levels, did not slow cognitive decline as compared with placebo over a period of 240 weeks in persons with preclinical Alzheimer's disease. (Funded by the National Institute on Aging and others; A4 ClinicalTrials.gov number, NCT02008357.).
Copyright © 2023 Massachusetts Medical Society.
Figures

Comment in
-
Treatments for AD: towards the right target at the right time.Nat Rev Neurol. 2023 Oct;19(10):581-582. doi: 10.1038/s41582-023-00869-0. Nat Rev Neurol. 2023. PMID: 37658242 No abstract available.
References
-
- Rowe CC, Ellis KA, Rimajova M, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 2010;31:1275–83. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R01 AG063689/AG/NIA NIH HHS/United States
- U24 AG057437/AG/NIA NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U19 AG010483/AG/NIA NIH HHS/United States
- U19AG010483/AG/NIA NIH HHS/United States
- P30 AG066508/AG/NIA NIH HHS/United States
- P30 AG062429/AG/NIA NIH HHS/United States
- P30 AG066462/AG/NIA NIH HHS/United States
- U01 AG010483/AG/NIA NIH HHS/United States
- K23 AG059888/AG/NIA NIH HHS/United States
- P30 AG072977/AG/NIA NIH HHS/United States
- P30 AG072947/AG/NIA NIH HHS/United States
- P30 AG072972/AG/NIA NIH HHS/United States
- P30 AG066514/AG/NIA NIH HHS/United States
- U24AG057437/AG/NIA NIH HHS/United States
- P30 AG072946/AG/NIA NIH HHS/United States
- R01AG063689/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
