Vedolizumab for induction and maintenance of remission in Crohn's disease
- PMID: 37458279
- PMCID: PMC10351211
- DOI: 10.1002/14651858.CD013611.pub2
Vedolizumab for induction and maintenance of remission in Crohn's disease
Abstract
Background: Vedolizumab blocks inflammatory activity within the gastrointestinal tract. Systematic reviews have demonstrated the efficacy of vedolizumab in ulcerative colitis and inflammatory bowel disease in general. This systematic review and meta-analysis summarises the current evidence of vedolizumab in the induction and maintenance of remission in Crohn's disease.
Objectives: To evaluate the benefits and harms of vedolizumab versus placebo for the induction and maintenance of remission in people with Crohn's disease.
Search methods: We used standard, extensive Cochrane search methods. The latest search date was 30 November 2022.
Selection criteria: We included randomised controlled trials (RCTs) and quasi-RCTs comparing vedolizumab to placebo for the induction or maintenance of remission in people with Crohn's disease.
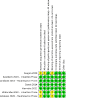
Data collection and analysis: We used standard Cochrane methods. For induction studies, the primary outcome was 1. clinical remission, and secondary outcomes were rates of 2. clinical response, 3. adverse events, 4. serious adverse events, 5. surgery, 6. endoscopic remission and 7. endoscopic response. For maintenance studies, the primary outcome was 1. maintenance of clinical remission, and secondary outcomes were rates of 2. adverse events, 3. serious adverse events, 4. surgery, 5. endoscopic remission and 6. endoscopic response. We used GRADE to assess certainty of evidence.
Main results: We analysed induction (4 trials, 1126 participants) and maintenance (3 trials, 894 participants) studies representing people across North America, Europe, Asia and Australasia separately. One maintenance trial administered subcutaneous vedolizumab whilst the other studies used the intravenous form. The mean age ranged between 32.6 and 38.6 years. Vedolizumab was superior to placebo for the induction of clinical remission (71 more per 1000 with clinical remission with vedolizumab; risk ratio (RR) 1.61, 95% confidence interval (CI) 1.20 to 2.17; number needed to treat for an additional beneficial outcome (NNTB) 13; 4 studies; high-certainty evidence) and superior to placebo for inducing clinical response (105 more per 1000 with clinical response with vedolizumab; RR 1.43, 95% CI 1.19 to 1.71; NNTB 8; 4 studies; high-certainty evidence). For the induction phase, vedolizumab may be equivalent to placebo for the development of serious adverse events (9 fewer serious adverse events per 1000 with vedolizumab; RR 0.91, 95% CI 0.62 to 1.33; 4 studies; low-certainty evidence) and probably equivalent to placebo for overall adverse events (6 fewer adverse events per 1000 with vedolizumab; RR 1.01, 95% CI 0.93 to 1.11; 4 studies; moderate-certainty evidence). Vedolizumab was superior to placebo for the maintenance of clinical remission (141 more per 1000 with maintenance of clinical remission with vedolizumab; RR 1.52, 95% CI 1.24 to 1.87; NNTB 7; 3 studies; high-certainty evidence). During the maintenance phase, vedolizumab may be equivalent to placebo for the development of serious adverse events (3 fewer serious adverse events per 1000 with vedolizumab; RR 0.98, 95% CI 0.68 to 1.39; 3 studies; low-certainty evidence) and probably equivalent to placebo for the development of overall adverse events (0 difference in adverse events per 1000; RR 1.00, 95% CI 0.94 to 1.07; 3 studies; moderate-certainty evidence).
Authors' conclusions: High-certainty data across four induction and three maintenance trials demonstrate that vedolizumab is superior to placebo in the induction and maintenance of remission in Crohn's disease. Overall adverse events are probably similar and serious adverse events may be similar between vedolizumab and placebo during both induction and maintenance phases of treatment. Head-to-head research comparing the efficacy and safety of vedolizumab to other biological therapies is required.
Copyright © 2023 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
SH: none.
VS: none.
MG: none.
AK: none.
GA was the Managing Editor of Cochrane Gut group. However, she has not been involved in any stage of the review's editorial process.
NSD has received a research grant from Takeda and GESA; speaking fees from Abbvie, Ferring, Shire and Pfizer; and is on an advisory board for Abbvie.
RKB: none.
Figures







Update of
- doi: 10.1002/14651858.CD013611
References
References to studies included in this review
Feagan 2008 {published data only}
-
- Feagan BG, Vandervoort MK, Greenberg GR, Wild G, Bitton A, Cohen A, et al. Treatment of active Crohn's disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clinical Gastroenterology and Hepatology 2008;6:1370-7. - PubMed
Sandborn 2013 – Induction Phase {published data only}
-
- Hanauer S, Colombel J-F, Feagan B, Rutgeerts P, Sandborn W, Sands B, et al. Vedolizumab maintenance therapy for Crohn's disease: results of GEMINI II, a randomized, placebo-controlled, double-blind, multicenter phase 3 trial. American Journal of Gastroenterology 2012;107:s620-1.
-
- Sandborn W, Sands B, Colombel J-F, Feagan B, Hanauer S, Rutgeerts P, et al. Vedolizumab induction therapy for Crohn's disease: results of GEMINI II, a randomized, placebo-controlled, double-blind, multicenter phase 3 trial. American Journal of Gastroenterology 2012;107:S624-5.
-
- Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. New England Journal of Medicine 2013;369(8):711-21. - PubMed
Sandborn 2013 – Maintenance Phase {published data only}
-
- Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. New England Journal of Medicine 2013;369(8):711-21. - PubMed
Sands 2014 {published data only}
-
- Sands B, Feagan B, Rutgeerts P, Colombel JF, Sandborn W, Sy R, et al. Effects of vedolizumab induction therapy for patients with Crohn's disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014;147(3):618-27. - PubMed
Vermeire 2021 {published data only}
-
- Vermeire S, D'Haens G, Baert F, Danese S, Kobayashi T, Loftus EV, et al. Efficacy and safety of vedolizumab subcutaneous formulation in patients with moderately to severely active Crohn's disease: results of the VISIBLE 2 study. Journal of Gastroenterology and Hepatology 2020;35(Suppl 1):115.
-
- Vermeire S, D'Haens GR, Baert FJ, Danese S. The visible 2 phase 3 study of efficacy and safety of vedolizumab SC for moderate-to-severe Crohn's disease. Gastroenterology 2020;158(6 (Suppl 1)):S-194.
Watanabe 2020 – Induction Phase {published data only}
-
- Ogata H, Motoya S, Watanabe K, Kanai T, Matsui T, Suzuki Y, et al. A phase 3 study of vedolizumab for induction and maintenance therapy in Japanese patients with moderate to severe Crohn's disease. Gastroenterology 2019;156(6 Suppl 1):S-1109.
-
- Ogata H, Motoya S, Watanabe K, Kanai T, Matsui T, Suzuki Y, et al. A phase 3 study of vedolizumab for induction and maintenance therapy in Japanese patients with moderate to severe Crohn's disease. Gastroenterology 2019;156(6):S-1109. [DOI: 10.1016/S0016-5085(19)39732-X] - DOI
Watanabe 2020 – Maintenance Phase {published data only}
References to studies excluded from this review
Pipek 2020 {published data only}
-
- Pipek B. Subcutaneous vedolizumab treatment for ulcerative colitis and Crohn‘s disease in clinical trial VISIBLE. Gastroenterologie a Hepatologie 2020;74(6):558-61. [DOI: 10.48095/ccgh2020558] - DOI
Sandborn 2014 {published data only}
-
- Sandborn WJ, Feagan B, Reinisch W, Smyth M, Xu J, Parikh A, et al. P497 Efficacy of continued vedolizumab therapy in patients with Crohn's disease who did not respond to vedolizumab induction therapy at week 6. Journal of Crohn's and Colitis 2014;8(Suppl 1):S274-5.
Vermeire 2017 {published data only}
-
- Vermeire S, Loftus EV Jr, Colombel JF, Feagan BG, Sandborn WJ, Sands BE, et al. Long-term efficacy of vedolizumab for Crohn's disease. Journal of Crohn's and Colitis 2017;11(4):412-24. - PubMed
Additional references
Bickston 2014
Bloomgren 2012
-
- Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. New England Journal of Medicine 2012;366(20):1870-80. - PubMed
Boyapati 2015
Boyapati 2016
-
- Boyapati RK, Kalla R, Satsangi J, Ho G. Biomarkers in search of precision medicine in IBD. American Journal of Gastroenterology 2016;111(12):1682-90. - PubMed
Butcher 1996
-
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science 1996;272(5258):60-6. - PubMed
D'Haens 2008
-
- D'Haens G, Baert F, Assche G, Caenepeel P, Vergauwe P, Tuynman H, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet 2008;371(9613):660-7. - PubMed
De Cruz 2013
-
- De Cruz P, Kamm MA, Prideaux L, Allen PB, Moore G. Mucosal healing in Crohn's disease: a systematic review. Inflammatory Bowel Diseases 2013;19(2):429-44. - PubMed
Eriksson 2017
-
- Eriksson C, Marsal J, Bergemalm D, Vigren L, Björk J, Eberhardson M, et al. Long-term effectiveness of vedolizumab in inflammatory bowel disease: a national study based on the Swedish National Quality Registry for Inflammatory Bowel Disease (SWIBREG). Scandinavian Journal of Gastroenterology 2017;52(6-7):722-9. - PubMed
Gordon 2021
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Hesterberg 1996
-
- Hesterberg PE, Winsor-Hines D, Briskin MJ, Soler-Ferran D, Merrill C, Mackay CR, et al. Rapid resolution of chronic colitis in the cotton-top tamarin with an antibody to a gut-homing integrin alpha 4 beta 7. Gastroenterology 1996;111(5):1373-80. - PubMed
Higgins 2017
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Irving 2021
-
- Irving PM, Sands BE, Hoops T, Izanec JL, Gao LL, Gasink C, et al. OP02 Ustekinumab versus adalimumab for induction and maintenance therapy in moderate-to-severe Crohn's disease: the SEAVUE study. Journal of Crohn's and Colitis 2021;15(Suppl 1):S001-2.
Lamb 2019
Lichtenstein 2018
-
- Lichtenstein G, Loftus E, Isaacs K, Regueiro MD, Gerson LB, Sands B. Clinical guideline: management of Crohn's disease in adults. American Journal of Gastroenterology 2018;113(4):481-517. - PubMed
Molodecky 2012
-
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142(1):46-54. - PubMed
Moćko 2016
RevMan Web 2020 [Computer program]
-
- Review Manager Web (RevMan Web). Version 1.22.0. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Shah 2016
-
- Shah SC, Colombel J-F, Sands BE, Narula N. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn's disease. Alimentary Pharmacology & Therapeutics 2016;43(3):317-33. - PubMed
Turner 2021
-
- Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021;160(5):1570-83. - PubMed
Vermeire 2017
-
- Vermeire S, Loftus E Jr, Colombel JF, Feagan BG, Sandborn WJ, Sands BC, et al. Long-term efficacy of vedolizumab for Crohn's disease. Journal of Crohn's and Colitis 2017;11(4):412-24. - PubMed
von Andrian 2003
-
- Andrian UH, Engelhardt B. α4 Integrins as therapeutic targets in autoimmune disease. New England Journal of Medicine 2003;348(1):68-72. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

