Gene coexpression network analysis reveals perirenal adipose tissue as an important target of prenatal malnutrition in sheep
- PMID: 37458462
- PMCID: PMC10642927
- DOI: 10.1152/physiolgenomics.00128.2022
Gene coexpression network analysis reveals perirenal adipose tissue as an important target of prenatal malnutrition in sheep
Abstract
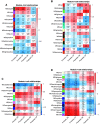
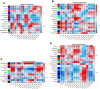
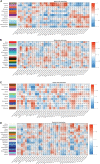
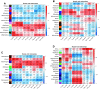
We have previously demonstrated that pre- and early postnatal malnutrition in sheep induced depot- and sex-specific changes in adipose morphological features, metabolic outcomes, and transcriptome in adulthood, with perirenal (PER) as the major target followed by subcutaneous (SUB) adipose tissue. We aimed to identify coexpressed and hub genes in SUB and PER to identify the underlying molecular mechanisms contributing to the early nutritional programming of adipose-related phenotypic outcomes. Transcriptomes of SUB and PER of male and female adult sheep with different pre- and early postnatal nutrition histories were used to construct networks of coexpressed genes likely to be functionally associated with pre- and early postnatal nutrition histories and phenotypic traits using weighted gene coexpression network analysis. The modules from PER showed enrichment of cell cycle regulation, gene expression, transmembrane transport, and metabolic processes associated with both sexes' prenatal nutrition. In SUB (only males), a module of enriched adenosine diphosphate metabolism and development correlated with prenatal nutrition. Sex-specific module enrichments were found in PER, such as chromatin modification in the male network but histone modification and mitochondria- and oxidative phosphorylation-related functions in the female network. These sex-specific modules correlated with prenatal nutrition and adipocyte size distribution patterns. Our results point to PER as a primary target of prenatal malnutrition compared to SUB, which played only a minor role. The prenatal programming of gene expression and cell cycle, potentially through epigenetic modifications, might be underlying mechanisms responsible for observed changes in PER expandability and adipocyte-size distribution patterns in adulthood in both sexes.
Keywords: WGCNA; early postnatal malnutrition; perirenal adipose tissue; prenatal malnutrition; subcutaneous adipose tissue.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
Similar articles
-
Depot and sex-specific implications for adipose tissue expandability and functional traits in adulthood of late prenatal and early postnatal malnutrition in a precocial sheep model.Physiol Rep. 2020 Oct;8(19):e14600. doi: 10.14814/phy2.14600. Physiol Rep. 2020. PMID: 33038074 Free PMC article.
-
Transcriptomics analysis of differentially expressed genes in subcutaneous and perirenal adipose tissue of sheep as affected by their pre- and early postnatal malnutrition histories.BMC Genomics. 2021 May 11;22(1):338. doi: 10.1186/s12864-021-07672-5. BMC Genomics. 2021. PMID: 33975549 Free PMC article.
-
Developmental programming: Adipose depot-specific transcriptional regulation by prenatal testosterone excess in a sheep model of PCOS.Mol Cell Endocrinol. 2021 Mar 1;523:111137. doi: 10.1016/j.mce.2020.111137. Epub 2020 Dec 25. Mol Cell Endocrinol. 2021. PMID: 33359827 Free PMC article.
-
Prenatal programming of postnatal obesity: fetal nutrition and the regulation of leptin synthesis and secretion before birth.Proc Nutr Soc. 2004 Aug;63(3):405-12. doi: 10.1079/pns2004370. Proc Nutr Soc. 2004. PMID: 15373950 Review.
-
Early-life programming of adipose tissue.Nutr Res Rev. 2020 Dec;33(2):244-259. doi: 10.1017/S0954422420000037. Epub 2020 Mar 2. Nutr Res Rev. 2020. PMID: 32115018 Review.
References
-
- Saxena A, Tiwari P, Wahi N, Soni A, Bansiwal RC, Kumar A, Sharma B, Punjabi P, Gupta N, Malik B, Medicherla KM, Suravajhala P, Mathur SK. Transcriptome profiling reveals association of peripheral adipose tissue pathology with type-2 diabetes in Asian Indians. Adipocyte 8: 125–136, 2019. doi:10.1080/21623945.2019.1595269. - DOI - PMC - PubMed
-
- Belligoli A, Compagnin C, Sanna M, Favaretto F, Fabris R, Busetto L, Foletto M, Dal Prà C, Serra R, Prevedello L, Da Re C, Bardini R, Mescoli C, Rugge M, Fioretto P, Conci S, Bettini S, Milan G, Vettor R. Characterization of subcutaneous and omental adipose tissue in patients with obesity and with different degrees of glucose impairment. Sci Rep 9: 1, 2019. doi:10.1038/s41598-019-47719-y. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

