Efficacy and safety of tonic motor activation (TOMAC) for medication-refractory restless legs syndrome: a randomized clinical trial
- PMID: 37458698
- PMCID: PMC10566236
- DOI: 10.1093/sleep/zsad190
Efficacy and safety of tonic motor activation (TOMAC) for medication-refractory restless legs syndrome: a randomized clinical trial
Abstract
Study objectives: The purpose of this study was to evaluate the efficacy and safety/tolerability of bilateral high-frequency tonic motor activation (TOMAC) in patients with medication-refractory restless legs syndrome (RLS).
Methods: RESTFUL was a multicenter, randomized, double-blind, sham-controlled trial in adults with medication-refractory moderate-to-severe primary RLS. Participants were randomized 1:1 to active or sham TOMAC for a double-blind, 4-week stage 1 and all received active TOMAC during open-label, 4-week stage 2. The primary endpoint was the Clinical Global Impressions-Improvement (CGI-I) responder rate at the end of stage 1. Key secondary endpoints included change to International RLS Study Group (IRLS) total score from study entry to the end of stage 1.
Results: A total of 133 participants were enrolled. CGI-I responder rate at the end of stage 1 was significantly greater for the active versus sham group (45% vs. 16%; Difference = 28%; 95% CI 14% to 43%; p = .00011). At the end of stage 2, CGI-I responder rate further increased to 61% for the active group. IRLS change at the end of stage 1 improved for the active versus sham group (-7.2 vs. -3.8; difference = -3.4; 95% CI -1.4 to -5.4; p = .00093). There were no severe or serious device-related adverse events (AEs). The most common AEs were mild discomfort and mild administration site irritation which resolved rapidly and reduced in prevalence over time.
Conclusions: TOMAC was safe, well tolerated, and reduced symptoms of RLS in medication-refractory patients. TOMAC is a promising new treatment for this population.
Clinical trial: Noninvasive Peripheral Nerve Stimulation for Medication-Refractory Primary RLS (The RESTFUL Study); clinicaltrials.gov/ct2/show/NCT04874155; Registered at ClinicalTrials.gov with the identifier number NCT04874155.
Keywords: bioelectronic; neurological disorder; neuromodulation; peripheral nerve stimulation; restless legs syndrome; sleep disorder.
© The Author(s) 2023. Published by Oxford University Press on behalf of Sleep Research Society.
Figures

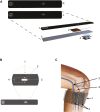
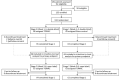
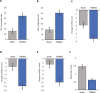
Comment in
-
Can tonic motor activation be the magical elixir for restless legs syndrome?Sleep. 2023 Oct 11;46(10):zsad230. doi: 10.1093/sleep/zsad230. Sleep. 2023. PMID: 37671678 Free PMC article. No abstract available.
References
-
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J; Restless Legs Syndrome Diagnosis and Epidemiology workshop at the National Institutes of Health. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101–119. doi:10.1016/s1389-9457(03)00010-8. - DOI - PubMed

