Smoking reduction using electronic nicotine delivery systems in combination with nicotine skin patches
- PMID: 37458789
- PMCID: PMC10471641
- DOI: 10.1007/s00213-023-06401-y
Smoking reduction using electronic nicotine delivery systems in combination with nicotine skin patches
Erratum in
-
Correction to: Smoking reduction using electronic nicotine delivery systems in combination with nicotine skin patches.Psychopharmacology (Berl). 2025 Jul 30. doi: 10.1007/s00213-025-06868-x. Online ahead of print. Psychopharmacology (Berl). 2025. PMID: 40736541 No abstract available.
Abstract
Rationale: Electronic nicotine delivery systems (ENDS) are used by smokers seeking to reduce combustible cigarette (CC) use, but the role of nicotine replacement vs. behavioral and sensory factors is still poorly understood. We hypothesized that providing nicotine from ENDS in addition to nicotine skin patches would promote smoking reduction relative to non-nicotine control ENDS.
Objectives: To assess the effects on smoking behavior of using nicotine vs. placebo ENDS in smokers using nicotine vs. placebo patches.
Methods: Ninety-four daily smokers were enrolled in a study that randomly assigned them to receive ENDS with nicotine vs. without nicotine and skin patches with vs. without nicotine. Smoking reduction and cessation were assessed over an 8-week period by self-report and by expired air carbon monoxide (CO) measurements. The primary outcome was defined as reduction in expired air CO.
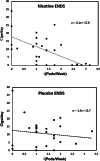
Results: The use of nicotine in ENDS led to significant reductions in smoking (ENDS nicotine vs. placebo difference in CO change = -9.2 ppm; 90% CI (-1.5 ppm, -16.9 ppm)) and was highly correlated with reductions in self-reported cigarettes per day (r=0.6). The effect of nicotine in nicotine patches was not statistically significant (patch nicotine vs. placebo difference in CO change = -0.1 ppm; 90% CI (-7.8 ppm, 7.6 ppm)).
Conclusions: The presence of nicotine in ENDS was associated with a large reduction in smoking. Additional studies will be needed to determine whether there may be additive effects of nicotine ENDS and nicotine patches on smoking abstinence.
Keywords: Cigarette; E-cigarette; ENDS; Electronic nicotine delivery system; Harm reduction; Nicotine patch; Smoking; Smoking cessation.
© 2023. The Author(s).
Conflict of interest statement
JER discloses research support from Foundation for a Smoke-Free World, Philip Morris International, Altria, Embera Neuro Therapeutics, Inc., Otsuka Pharmaceutical, JUUL Labs, consulting with Revive pharmaceuticals, and consulting and patent purchase agreements with Philip Morris International.
JMD discloses financial support from Predictably Human Inc., including funding for research, consulting fees, and equity. Predictably Human Inc. is focused on development of a prescription treatment for smoking and e-cigarette use.
Figures

References
-
- Adriaens K, Belmans E, Van Gucht D, Baeyens F (2021) Electronic cigarettes in standard smoking cessation treatment by tobacco counselors in Flanders: e-cigarette users show similar if not higher quit rates as those using commonly recommended smoking cessation aids. Harm Reduct J 18(1):28. 10.1186/s12954-021-00475-7 - PMC - PubMed
-
- Benowitz NL, Zevin S, Jacob P (1998) 3rd Suppression of nicotine intake during ad libitum cigarette smoking by high-dose transdermal nicotine. J Pharmacol Exp Ther 287(3):958–962 - PubMed
-
- Blondal T, Gudmundsson LJ, Olafsdottir I, Gustavsson G, Westin A (1999) Nicotine nasal spray with nicotine patch for smoking cessation: randomised trial with six year follow up. BMJ 318(7179):285–288. 10.1136/bmj.318.7179.285 Erratum in: BMJ 1999 Mar 20;318(7186):764. PMID: 9924052; PMCID: PMC27708 - PMC - PubMed
-
- Callahan-Lyon P, Sipes G (2018) Tobacco product or medical product? Presentation at the Society for Research on Nicotine & Tobacco’s Annual Meeting, held February 21, 2018 in Baltimore, Maryland. https://www.fda.gov/media/118946/download
-
- Carter BD, Abnet CC, Feskanich D, Freedman ND, Hartge P, Lewis CE, Ockene JK, Prentice RL, Speizer FE, Thun MJ, Jacobs EJ (2015) Smoking and mortality—beyond established causes. NEJM 372(7):631–640. 10.1056/NEJMsa1407211 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

