Targeting mitochondria in the aged cerebral vasculature with SS-31, a proteomic study of brain microvessels
- PMID: 37458933
- PMCID: PMC10643806
- DOI: 10.1007/s11357-023-00845-y
Targeting mitochondria in the aged cerebral vasculature with SS-31, a proteomic study of brain microvessels
Abstract
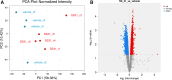
Cognitive impairment and dementias during aging such as Alzheimer's disease are linked to functional decline and structural alterations of the brain microvasculature. Although mechanisms leading to microvascular changes during aging are not clear, loss of mitochondria, and reduced efficiency of remaining mitochondria appear to play a major role. Pharmacological agents, such as SS-31, which target mitochondria have been shown to be effective during aging and diseases; however, the benefit to mitochondrial- and non-mitochondrial proteins in the brain microvasculature has not been examined. We tested whether attenuation of aging-associated changes in the brain microvascular proteome via targeting mitochondria represents a therapeutic option for the aging brain. We used aged male (> 18 months) C57Bl6/J mice treated with a mitochondria-targeted tetrapeptide, SS-31, or vehicle saline. Cerebral blood flow (CBF) was determined using laser speckle imaging during a 2-week treatment period. Then, isolated cortical microvessels (MVs) composed of end arterioles, capillaries, and venules were used for Orbitrap Eclipse Tribrid mass spectrometry. CBF was similar among the groups, whereas bioinformatic analysis revealed substantial differences in protein abundance of cortical MVs between SS-31 and vehicle. We identified 6267 proteins, of which 12% were mitochondria-associated. Of this 12%, 107 were significantly differentially expressed and were associated with oxidative phosphorylation, metabolism, the antioxidant defense system, or mitochondrial dynamics. Administration of SS-31 affected many non-mitochondrial proteins. Our findings suggest that mitochondria in the microvasculature represent a therapeutic target in the aging brain, and widespread changes in the proteome may underlie the rejuvenating actions of SS-31 in aging.
Keywords: Aging; Brain microvasculature; Mitochondria; Proteomics.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Nyul-Toth A, Tarantini S, DelFavero J, Yan F, Balasubramanian P, Yabluchanskiy A, et al. Demonstration of age-related blood-brain barrier disruption and cerebromicrovascular rarefaction in mice by longitudinal intravital two-photon microscopy and optical coherence tomography. Am J Physiol Heart Circ Physiol. 2021;320(4):H1370–H92. doi: 10.1152/ajpheart.00709.2020. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

