Extracellular Vesicle-Serpine-1 Affects Neural Progenitor Cell Mitochondrial Networks and Synaptic Density: Modulation by Amyloid Beta and HIV-1
- PMID: 37458985
- PMCID: PMC10533645
- DOI: 10.1007/s12035-023-03456-y
Extracellular Vesicle-Serpine-1 Affects Neural Progenitor Cell Mitochondrial Networks and Synaptic Density: Modulation by Amyloid Beta and HIV-1
Abstract
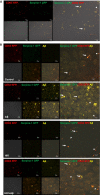
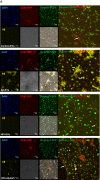
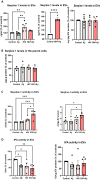
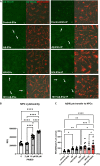
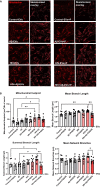
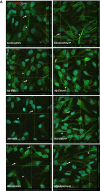
Brain endothelial extracellular vesicles carrying amyloid beta (EV-Aβ) can be transferred to neural progenitor cells (NPCs) leading to NPC dysfunction. However, the events involved in this EV-mediated Aβ pathology are unclear. EV-proteomics studies identified Serpine-1 (plasminogen activator inhibitor 1, PAI-1) as a major connecting "hub" on several protein-protein interaction maps. Serpine-1 was described as a key player in Aβ pathology and was linked to HIV-1 infection as well. Therefore, the aim of this work was to address the hypothesis that Serpine-1 can be transferred via EVs from brain endothelial cells (HBMEC) to NPCs and contribute to NPC dysfunction. HBMEC concentrated and released Serpine-1 via EVs, the effect that was potentiated by HIV-1 and Aβ. EVs loaded with Serpine-1 were readily taken up by NPCs, and HIV-1 enhanced this event. Interestingly, a highly specific Serpine-1 inhibitor PAI039 increased EV-Aβ transfer to NPCs in the presence of HIV-1. PAI039 also partially blocked mitochondrial network morphology alterations in the recipient NPCs, which developed mainly after HIV + Aβ-EV transfer. PAI039 partly attenuated HIV-EV-mediated decreased synaptic protein levels in NPCs, while increased synaptic protein levels in NPC projections. These findings contribute to a better understanding of the complex mechanisms underlying EV-Serpine-1 related Aβ pathology in the context of HIV infection. They are relevant to HIV-1 associated neurocognitive disorders (HAND) in an effort to elucidate the mechanisms of neuropathology in HIV infection.
Keywords: Amyloid beta; Blood–brain barrier; Extracellular vesicles; HIV-1; Neural progenitor cells; Serpine-1.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







Update of
-
Extracellular vesicle-Serpine-1 affects neural progenitor cell mitochondrial functions and synaptic density: modulation by amyloid beta and HIV-1.Res Sq [Preprint]. 2023 Feb 15:rs.3.rs-2551245. doi: 10.21203/rs.3.rs-2551245/v1. Res Sq. 2023. Update in: Mol Neurobiol. 2023 Nov;60(11):6441-6465. doi: 10.1007/s12035-023-03456-y. PMID: 36824983 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

