Effectiveness and Costs of Molecular Screening and Treatment for Bacterial Vaginosis to Prevent Preterm Birth: The AuTop Randomized Clinical Trial
- PMID: 37459059
- PMCID: PMC10352927
- DOI: 10.1001/jamapediatrics.2023.2250
Effectiveness and Costs of Molecular Screening and Treatment for Bacterial Vaginosis to Prevent Preterm Birth: The AuTop Randomized Clinical Trial
Abstract
Importance: Bacterial vaginosis (BV) is a well-known risk factor for preterm birth. Molecular diagnosis of BV is now available. Its impact in the screening and treatment of BV during pregnancy on preterm births has not been evaluated to date.
Objective: To evaluate the clinical and economic effects of point-of-care quantitative real-time polymerase chain reaction screen and treat for BV in low-risk pregnant women on preterm birth.
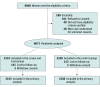
Design, setting, and participants: The AuTop trial was a prospective, multicenter, parallel, individually randomized, open-label, superiority trial conducted in 19 French perinatal centers between March 9, 2015, and December 18, 2017. Low-risk pregnant women before 20 weeks' gestation without previous preterm births or late miscarriages were enrolled. Data were analyzed from October 2021 to November 2022.
Interventions: Participants were randomized 1:1 to BV screen and treat using self-collected vaginal swabs (n = 3333) or usual care (n = 3338). BV was defined as Atopobium vaginae (Fannyhessea vaginae) load of 108 copies/mL or greater and/or Gardnerella vaginalis load of 109 copies/mL or greater, using point-of-care quantitative real-time polymerase chain reaction assays. The control group received usual care with no screening of BV.
Main outcomes and measures: Overall rate of preterm birth before 37 weeks' gestation and total costs were calculated in both groups. Secondary outcomes were related to treatment success as well as maternal and neonate health. Post hoc subgroup analyses were conducted.
Results: Among 6671 randomized women (mean [SD] age, 30.6 [5.0] years; mean [SD] gestational age, 15.5 [2.8] weeks), the intention-to-treat analysis of the primary clinical and economic outcomes showed no evidence of a reduction in the rate of preterm birth and total costs with the screen and treat strategy compared with usual care. The rate of preterm birth was 3.8% (127 of 3333) in the screen and treat group and 4.6% (153 of 3338) in the control group (risk ratio [RR], 0.83; 95% CI, 0.66-1.05; P = .12). On average, the cost of the intervention was €203.6 (US $218.0) per participant, and the total average cost was €3344.3 (US $3580.5) in the screen and treat group vs €3272.9 (US $3504.1) in the control group, with no significant differences being observed. In the subgroup of nulliparous women (n = 3438), screen and treat was significantly more effective than usual care (RR, 0.62; 95% CI, 0.45-0.84; P for interaction = .003), whereas no statistical difference was found in multiparous (RR, 1.30; 95% CI, 0.90-1.87).
Conclusion and relevance: In this clinical trial of pregnant women at low risk of preterm birth, molecular screening and treatment for BV based on A vaginae (F vaginae) and/or G vaginalis quantification did not significantly reduce preterm birth rates. Post hoc analysis suggests a benefit of screen and treat in low-risk nulliparous women, warranting further evaluation in this group.
Trial registration: ClinicalTrials.gov Identifier: NCT02288832.
Conflict of interest statement
Figures
Comment in
-
Further Considerations Regarding Molecular Screening and Treatment of Bacterial Vaginosis.JAMA Pediatr. 2024 Jan 1;178(1):93. doi: 10.1001/jamapediatrics.2023.4729. JAMA Pediatr. 2024. PMID: 37930686 No abstract available.
-
Further Considerations Regarding Molecular Screening and Treatment of Bacterial Vaginosis.JAMA Pediatr. 2024 Jan 1;178(1):93-94. doi: 10.1001/jamapediatrics.2023.4732. JAMA Pediatr. 2024. PMID: 37930687 No abstract available.
-
Further Considerations Regarding Molecular Screening and Treatment of Bacterial Vaginosis.JAMA Pediatr. 2024 Jan 1;178(1):94-95. doi: 10.1001/jamapediatrics.2023.4755. JAMA Pediatr. 2024. PMID: 37930708 No abstract available.
References
-
- Menard JP, Fenollar F, Raoult D, Boubli L, Bretelle F. Self-collected vaginal swabs for the quantitative real-time polymerase chain reaction assay of Atopobium vaginae and Gardnerella vaginalis and the diagnosis of bacterial vaginosis. Eur J Clin Microbiol Infect Dis. 2012;31(4):513-518. doi:10.1007/s10096-011-1341-8 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical