Efficacy of a Clinical Decision Rule to Enable Direct Oral Challenge in Patients With Low-Risk Penicillin Allergy: The PALACE Randomized Clinical Trial
- PMID: 37459086
- PMCID: PMC10352926
- DOI: 10.1001/jamainternmed.2023.2986
Efficacy of a Clinical Decision Rule to Enable Direct Oral Challenge in Patients With Low-Risk Penicillin Allergy: The PALACE Randomized Clinical Trial
Abstract
Importance: Fewer than 5% of patients labeled with a penicillin allergy are truly allergic. The standard of care to remove the penicillin allergy label in adults is specialized testing involving prick and intradermal skin testing followed by an oral challenge with penicillin. Skin testing is resource intensive, limits practice to specialist-trained physicians, and restricts the global population who could undergo penicillin allergy delabeling.
Objective: To determine whether a direct oral penicillin challenge is noninferior to the standard of care of penicillin skin testing followed by an oral challenge in patients with a low-risk penicillin allergy.
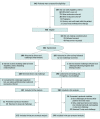
Design, setting, and participants: This parallel, 2-arm, noninferiority, open-label, multicenter, international randomized clinical trial occurred in 6 specialized centers, 3 in North America (US and Canada) and 3 in Australia, from June 18, 2021, to December 2, 2022. Eligible adults had a PEN-FAST score lower than 3. PEN-FAST is a prospectively derived and internationally validated clinical decision rule that enables point-of-care risk assessment for adults reporting penicillin allergies.
Interventions: Patients were randomly assigned to either direct oral challenge with penicillin (intervention arm) or a standard-of-care arm of penicillin skin testing followed by oral challenge with penicillin (control arm).
Main outcome and measure: The primary outcome was a physician-verified positive immune-mediated oral penicillin challenge within 1 hour postintervention in the intention-to-treat population. Noninferiority was achieved if a 1-sided 95% CI of the risk difference (RD) did not exceed 5 percentage points (pp).
Results: A total of 382 adults were randomized, with 377 patients (median [IQR] age, 51 [35-65] years; 247 [65.5%] female) included in the analysis: 187 in the intervention group and 190 in the control group. Most patients had a PEN-FAST score of 0 or 1. The primary outcome occurred in 1 patient (0.5%) in the intervention group and 1 patient (0.5%) in the control group, with an RD of 0.0084 pp (90% CI, -1.22 to 1.24 pp). The 1-sided 95% CI was below the noninferiority margin of 5 pp. In the 5 days following the oral penicillin challenge, 9 immune-mediated adverse events were recorded in the intervention group and 10 in the control group (RD, -0.45 pp; 95% CI, -4.87 to 3.96 pp). No serious adverse events occurred.
Conclusions and relevance: In this randomized clinical trial, direct oral penicillin challenge in patients with a low-risk penicillin allergy was noninferior compared with standard-of-care skin testing followed by oral challenge. In patients with a low-risk history, direct oral penicillin challenge is a safe procedure to facilitate the removal of a penicillin allergy label.
Trial registration: ClinicalTrials.gov Identifier: NCT04454229.
Conflict of interest statement
Figures
Comment in
-
The Penicillin Allergy Decision Rule-Something New for Penicillin Allergy.JAMA Intern Med. 2023 Sep 1;183(9):953-954. doi: 10.1001/jamainternmed.2023.3936. JAMA Intern Med. 2023. PMID: 37548988 No abstract available.
-
In low-risk penicillin allergies, direct oral challenge was noninferior to skin testing followed by oral challenge.Ann Intern Med. 2023 Nov;176(11):JC127. doi: 10.7326/J23-0085. Epub 2023 Nov 7. Ann Intern Med. 2023. PMID: 37931260
References
-
- Trubiano JA, Chen C, Cheng AC, Grayson ML, Slavin MA, Thursky KA; National Antimicrobial Prescribing Survey (NAPS) . Antimicrobial allergy ‘labels’ drive inappropriate antimicrobial prescribing: lessons for stewardship. J Antimicrob Chemother. 2016;71(6):1715-1722. doi: 10.1093/jac/dkw008 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

