Effects of Using Different Indirect Techniques on the Calculation of Reference Intervals: Observational Study
- PMID: 37459170
- PMCID: PMC10390978
- DOI: 10.2196/45651
Effects of Using Different Indirect Techniques on the Calculation of Reference Intervals: Observational Study
Abstract
Background: Reference intervals (RIs) play an important role in clinical decision-making. However, due to the time, labor, and financial costs involved in establishing RIs using direct means, the use of indirect methods, based on big data previously obtained from clinical laboratories, is getting increasing attention. Different indirect techniques combined with different data transformation methods and outlier removal might cause differences in the calculation of RIs. However, there are few systematic evaluations of this.
Objective: This study used data derived from direct methods as reference standards and evaluated the accuracy of combinations of different data transformation, outlier removal, and indirect techniques in establishing complete blood count (CBC) RIs for large-scale data.
Methods: The CBC data of populations aged ≥18 years undergoing physical examination from January 2010 to December 2011 were retrieved from the First Affiliated Hospital of China Medical University in northern China. After exclusion of repeated individuals, we performed parametric, nonparametric, Hoffmann, Bhattacharya, and truncation points and Kolmogorov-Smirnov distance (kosmic) indirect methods, combined with log or BoxCox transformation, and Reed-Dixon, Tukey, and iterative mean (3SD) outlier removal methods in order to derive the RIs of 8 CBC parameters and compared the results with those directly and previously established. Furthermore, bias ratios (BRs) were calculated to assess which combination of indirect technique, data transformation pattern, and outlier removal method is preferrable.
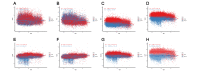
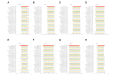
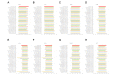
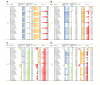
Results: Raw data showed that the degrees of skewness of the white blood cell (WBC) count, platelet (PLT) count, mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and mean corpuscular volume (MCV) were much more obvious than those of other CBC parameters. After log or BoxCox transformation combined with Tukey or iterative mean (3SD) processing, the distribution types of these data were close to Gaussian distribution. Tukey-based outlier removal yielded the maximum number of outliers. The lower-limit bias of WBC (male), PLT (male), hemoglobin (HGB; male), MCH (male/female), and MCV (female) was greater than that of the corresponding upper limit for more than half of 30 indirect methods. Computational indirect choices of CBC parameters for males and females were inconsistent. The RIs of MCHC established by the direct method for females were narrow. For this, the kosmic method was markedly superior, which contrasted with the RI calculation of CBC parameters with high |BR| qualification rates for males. Among the top 10 methodologies for the WBC count, PLT count, HGB, MCV, and MCHC with a high-BR qualification rate among males, the Bhattacharya, Hoffmann, and parametric methods were superior to the other 2 indirect methods.
Conclusions: Compared to results derived by the direct method, outlier removal methods and indirect techniques markedly influence the final RIs, whereas data transformation has negligible effects, except for obviously skewed data. Specifically, the outlier removal efficiency of Tukey and iterative mean (3SD) methods is almost equivalent. Furthermore, the choice of indirect techniques depends more on the characteristics of the studied analyte itself. This study provides scientific evidence for clinical laboratories to use their previous data sets to establish RIs.
Keywords: clinical; clinical decision-making; comparative study; complete blood count; data transformation; indirect method; laboratory; outliers; platelets; red blood cells; reference interval; white blood cells.
©Dan Yang, Zihan Su, Runqing Mu, Yingying Diao, Xin Zhang, Yusi Liu, Shuo Wang, Xu Wang, Lei Zhao, Hongyi Wang, Min Zhao. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 17.07.2023.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
Similar articles
-
Complete blood count reference intervals and age- and sex-related trends of North China Han population.Clin Chem Lab Med. 2014 Jul;52(7):1025-32. doi: 10.1515/cclm-2012-0486. Clin Chem Lab Med. 2014. PMID: 24497225
-
Reference intervals of complete blood count parameters in the adult western Sudanese population.BMC Res Notes. 2024 Apr 2;17(1):99. doi: 10.1186/s13104-024-06754-3. BMC Res Notes. 2024. PMID: 38566261 Free PMC article.
-
Reference Intervals of Red Blood Cell Parameters in Healthy Adults of the Chinese Population in High-Altitude Areas.Clin Lab. 2025 Aug 1;71(8). doi: 10.7754/Clin.Lab.2024.240734. Clin Lab. 2025. PMID: 40779459
-
Hematological parameters' reference intervals in apparently healthy individuals in Saudi Arabia: a systematic review and meta-analysis.Front Med (Lausanne). 2025 Apr 17;12:1522492. doi: 10.3389/fmed.2025.1522492. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40313540 Free PMC article.
-
Development of next-generation reference interval models to establish reference intervals based on medical data: current status, algorithms and future consideration.Crit Rev Clin Lab Sci. 2024 Jun;61(4):298-316. doi: 10.1080/10408363.2023.2291379. Epub 2023 Dec 26. Crit Rev Clin Lab Sci. 2024. PMID: 38146650 Review.
Cited by
-
Study on the Applicability of WHO Serum Iodine Standards for Normal Individuals in the Chinese Population-A Cross-sectional Study from Six Provinces in China.Biol Trace Elem Res. 2025 Feb 21. doi: 10.1007/s12011-025-04549-6. Online ahead of print. Biol Trace Elem Res. 2025. PMID: 39982609
-
Insulin reference intervals in Brazilian adolescents by direct and indirect approaches: validation of a data mining method from laboratory data.J Pediatr (Rio J). 2024 Sep-Oct;100(5):512-518. doi: 10.1016/j.jped.2024.03.009. Epub 2024 Apr 23. J Pediatr (Rio J). 2024. PMID: 38670169 Free PMC article.
References
-
- Hickman PE, Koerbin G, Potter JM, Glasgow N, Cavanaugh JA, Abhayaratna WP, West NP, Glasziou P. Choice of statistical tools for outlier removal causes substantial changes in analyte reference intervals in healthy populations. Clin Chem. 2020 Dec 01;66(12):1558–1561. doi: 10.1093/clinchem/hvaa208.5937292 - DOI - PubMed
-
- Clinical and Laboratory Standards Institute . Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline—Third Edition. CLSI Document EP28-A3c. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
-
- Jones GRD, Haeckel R, Loh TP, Sikaris K, Streichert T, Katayev A, Barth JH, Ozarda Y, IFCC Committee on Reference Intervals and Decision Limits Indirect methods for reference interval determination - review and recommendations. Clin Chem Lab Med. 2018 Dec 19;57(1):20–29. doi: 10.1515/cclm-2018-0073. https://www.degruyter.com/document/doi/10.1515/cclm-2018-0073 /j/cclm.ahead-of-print/cclm-2018-0073/cclm-2018-0073.xml - DOI - DOI - PubMed
-
- Ozarda Y, Ichihara K, Jones G, Streichert T, Ahmadian R, IFCC Committee on Reference Intervals and Decision Limits Comparison of reference intervals derived by direct and indirect methods based on compatible datasets obtained in Turkey. Clin Chim Acta. 2021 Sep;520:186–195. doi: 10.1016/j.cca.2021.05.030.S0009-8981(21)00186-8 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

