What's the Catch? The Significance of Catch Bonds in T Cell Activation
- PMID: 37459191
- PMCID: PMC10732538
- DOI: 10.4049/jimmunol.2300141
What's the Catch? The Significance of Catch Bonds in T Cell Activation
Abstract
One of the main goals in T cell biology has been to investigate how TCR recognition of peptide:MHC (pMHC) determines T cell phenotype and fate. Ag recognition is required to facilitate survival, expansion, and effector function of T cells. Historically, TCR affinity for pMHC has been used as a predictor for T cell fate and responsiveness, but there have now been several examples of nonfunctional high-affinity clones and low-affinity highly functional clones. Recently, more attention has been paid to the TCR being a mechanoreceptor where the key biophysical determinant is TCR bond lifetime under force. As outlined in this review, the fundamental parameters between the TCR and pMHC that control Ag recognition and T cell triggering are affinity, bond lifetime, and the amount of force at which the peak lifetime occurs.
Copyright © 2023 by The American Association of Immunologists, Inc.
Conflict of interest statement
Figures

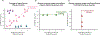
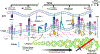
References
-
- Kane LP, Lin J, and Weiss A. 2000. Signal transduction by the TCR for antigen. Curr Opin Immunol 12: 242–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

