Long-term sutimlimab improves quality of life for patients with cold agglutinin disease: CARDINAL 2-year follow-up
- PMID: 37459203
- PMCID: PMC10558612
- DOI: 10.1182/bloodadvances.2022009318
Long-term sutimlimab improves quality of life for patients with cold agglutinin disease: CARDINAL 2-year follow-up
Erratum in
-
Röth A, Broome CM, Barcellini W, et al. Long-term sutimlimab improves quality of life for patients with cold agglutinin disease: CARDINAL 2-year follow-up. Blood Adv. 2023;7(19):5890-5897.Blood Adv. 2024 Jun 11;8(11):2650. doi: 10.1182/bloodadvances.2024013366. Blood Adv. 2024. PMID: 38809539 Free PMC article. No abstract available.
Abstract
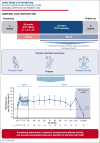
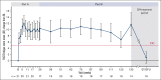
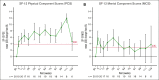
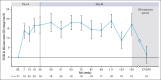
Cold agglutinin disease (CAD) is a rare form of autoimmune hemolytic anemia with a substantial burden on patient's quality of life. CARDINAL was a 2-part, open-label, single-arm, multicenter phase 3 study evaluating the C1s inhibitor, sutimlimab, for treatment of CAD. Part A consisted of the pivotal study phase, with the part B extension phase assessing long-term safety and durability of response including patient-reported outcomes, which is the focus of this report. Altogether, 22 patients continued from part A to part B, majority female (68.2%) with a median age of 71.5 years (range, 55-85). Throughout treatment, score improvement on the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scale exceeded a predefined, group-level clinically important change of ≥5 points vs baseline, with a mean (standard error [SE]) change of 11.7 (3.7) points at week 135. The 12-Item Short Form Health Survey physical and mental component scores remained above baseline, with week 123 mean change (SE) exceeding clinically important changes of 3.9 for physical and 2.8 for mental component scores at 4.7 (2.8) and 3.8 (5.7) points, respectively. EuroQol Visual Analogue Scale, scoring patients' self-rated health, also remained above baseline with a change of 17.1 (5.6) points at week 135. Patient Global Impression of (fatigue) Severity improved vs baseline, corroborating FACIT-Fatigue scores. Patient Global Impression of Change indicated a reduction in perceived disease burden. Data from CARDINAL part B support sustained alleviation of CAD disease burden after long-term treatment with sutimlimab over 2 years, returning toward baseline upon treatment cessation. This trial was registered at www.clinicaltrials.gov as #NCT03347396.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.R. has received research support from Roche, and received honoraria and provided consultancy to Alexion Pharmaceuticals Inc, Amgen, Apellis Pharmaceuticals, Biocryst, Grifols, Kira, Novartis, Roche, Bioverativ (a Sanofi company), Sanofi, and Sobi. C.M.B. has received honoraria and/or research funding from Alexion Pharmaceuticals, Bioverativ, Cellphire, Incyte, Rigel, and Sanofi Genzyme. W.B. has received research support from Alexion Pharmaceuticals and Novartis; participated in advisory boards for Agios, Alexion Pharmaceuticals Inc, Bioverativ, and Incyte; and has been an invited speaker for Alexion Pharmaceuticals Inc, and Novartis. T.H.A.T. has participated in advisory boards for Janssen, Novartis, and Sobi. S.D. has received grant funding, honoraria, and/or speaker’s fees from Janssen, BeiGene, and Sanofi. D.C. is President of
Figures

References
-
- Berentsen S, Ulvestad E, Langholm R, et al. Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematologica. 2006;91(4):460–466. - PubMed
-
- Berentsen S. Complement activation and inhibition in autoimmune hemolytic anemia: focus on cold agglutinin disease. Semin Hematol. 2018;55(3):141–149. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

