Post- and Pre-Radiolabeling Assays for anti Thymidine Cyclobutane Dimers as Intrinsic Photoprobes of Various Types of G-Quadruplexes, Reverse Hoogsteen Hairpins, and Other Non-B DNA Structures
- PMID: 37459251
- PMCID: PMC10474795
- DOI: 10.1021/acs.biochem.3c00155
Post- and Pre-Radiolabeling Assays for anti Thymidine Cyclobutane Dimers as Intrinsic Photoprobes of Various Types of G-Quadruplexes, Reverse Hoogsteen Hairpins, and Other Non-B DNA Structures
Abstract
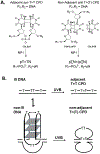
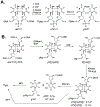
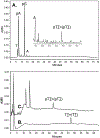
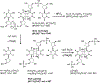
G-quadruplexes are thought to play an important role in gene regulation and telomere maintenance, but developing probes for their presence and location is challenging due to their transitory and highly dynamic nature. The majority of probes for G-quadruplexes have relied on antibody or small-molecule binding agents, many of which can also alter the dynamics and relative populations of G-quadruplexes. Recently, it was discovered that ultraviolet B (UVB) irradiation of human telomeric DNA and various G-quadruplex forming sequences found in human promoters, as well as reverse Hoogsteen hairpins, produces a unique class of non-adjacent anti cyclobutane pyrimidine dimers (CPDs). Therefore, one can envision using a pulse of UVB light to irreversibly trap these non-B DNA structures via anti CPD formation without perturbing their dynamics, after which the anti CPDs can be identified and mapped. As a first step toward this goal, we report radioactive post- and pre-labeling assays for the detection of non-adjacent CPDs and illustrate their use in detecting trans,anti T=(T) CPD formation in a human telomeric DNA sequence. Both assays make use of snake venom phosphodiesterase (SVP) to degrade the trans,anti T=(T) CPD-containing DNA to the tetranucleotide pT
Conflict of interest statement
All authors declare no competing financial interest.
Figures





Similar articles
-
Photocrosslinking of G-Quadruplex-Forming Sequences found in Human Promoters.Photochem Photobiol. 2019 Jan;95(1):252-266. doi: 10.1111/php.12991. Epub 2018 Sep 26. Photochem Photobiol. 2019. PMID: 30084501 Free PMC article.
-
Evidence for Reverse Hoogsteen Hairpin Intermediates in the Photocrosslinking of Human Telomeric DNA Sequences.Photochem Photobiol. 2018 Jul;94(4):685-697. doi: 10.1111/php.12898. Epub 2018 Mar 31. Photochem Photobiol. 2018. PMID: 29418001 Free PMC article.
-
Conformational and electronic effects on the formation of anti cyclobutane pyrimidine dimers in G-quadruplex structures.Phys Chem Chem Phys. 2017 Jan 25;19(4):3325-3336. doi: 10.1039/c6cp05604k. Phys Chem Chem Phys. 2017. PMID: 28091673
-
Adjacent and Nonadjacent Dipyrimidine Photoproducts as Intrinsic Probes of DNA Secondary and Tertiary Structure†.Photochem Photobiol. 2023 Mar;99(2):277-295. doi: 10.1111/php.13694. Epub 2022 Oct 6. Photochem Photobiol. 2023. PMID: 35980594 Review.
-
Solar UV radiation-induced DNA Bipyrimidine photoproducts: formation and mechanistic insights.Top Curr Chem. 2015;356:249-75. doi: 10.1007/128_2014_553. Top Curr Chem. 2015. PMID: 25370518 Review.
References
-
- Sinden RR Slipped strand DNA structures. Front. Biosci 2007, 12, 4788. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

