Efficacy of probiotics and trimebutine maleate for abemaciclib-induced diarrhea: A randomized, open-label phase II trial (MERMAID, WJOG11318B)
- PMID: 37459790
- PMCID: PMC10512094
- DOI: 10.1016/j.breast.2023.07.003
Efficacy of probiotics and trimebutine maleate for abemaciclib-induced diarrhea: A randomized, open-label phase II trial (MERMAID, WJOG11318B)
Abstract
Background: Abemaciclib-induced diarrhea (AID) impairs quality of life (QOL) and treatment adherence in patients with breast cancer. Supportive treatment with loperamide is associated with constipation. We hypothesized that probiotics and trimebutine maleate (TM) would decrease the frequency of AID without causing constipation.
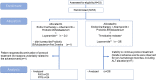
Methods: Hormone receptor-positive, human epidermal growth factor 2-negative advanced breast cancer patients were randomized into the probiotic Bifidobacterium (A) or probiotic Bifidobacterium and TM (B) groups. Endocrine therapy, Abemaciclib and probiotic Bifidobacterium three times a day for 28 days, was administered to both arms. Arm B was treated with TM upon the onset of diarrhea. The primary endpoint was the percentage of patients who experienced grade ≥2 diarrhea. The secondary endpoints were safety, frequency, and duration of all-grade diarrhea; frequency of emesis and constipation; usage of loperamide; and health-related QOL/patient-reported outcome during the study. We evaluated whether the primary endpoint of each arm exceeded the predetermined threshold.
Results: Fifty-one patients completed treatment. Grade 2 diarrhea occurred in 52% and 50% of patients in Arm A and Arm B, respectively. One patient experienced grade 3 diarrhea in each arm. The median duration of grade2 diarrhea was 2 and 2.5day, and only one patient required dose reduction. Grade ≥2 constipation was observed in 4% of Arm A and 3.6% of Arm B.
Conclusions: Probiotic Bifidobacterium or the combination of probiotic Bifidobacterium with TM did not decrease the incidence of grade 2 or greater diarrhea compared with historical control, although the grade 3 or greater diarrhea was reduced.
Clinical trial registration: jRCT (Japan registry of clinical trials). jRCTs031190154.
Keywords: CDK4/6 inhibitor; Constipation; Diarrhea; Probiotics; Quality of life.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Tripathy D., Im S.A., Colleoni M., Franke F., Bardia A., Harbeck N., et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19:904–915. - PubMed
-
- Finn R.S., Martin M., Rugo H.S., Jones S., Im S.A., Gelmon K., et al. Palbociclib and Letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. - PubMed
-
- Goetz M.P., Toi M., Campone M., Sohn J., Paluch-Shimon S., Huober J., et al. Monarch 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–3646. - PubMed
-
- Burstein H.J., Somerfield M.R., Barton D.L., Dorris A., Fallowfield L.J., Jain D., et al. Endocrine treatment and targeted therapy for hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer: ASCO guideline update. J Clin Oncol. 2021;39:3959–3977. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

