IN.PACT AV Access Randomized Trial of Drug-Coated Balloons for Dysfunctional Arteriovenous Fistulae: Clinical Outcomes through 36 Months
- PMID: 37460061
- PMCID: PMC11702510
- DOI: 10.1016/j.jvir.2023.07.007
IN.PACT AV Access Randomized Trial of Drug-Coated Balloons for Dysfunctional Arteriovenous Fistulae: Clinical Outcomes through 36 Months
Abstract
Purpose: To present the 36-month outcomes of the prospective randomized IN.PACT AV Access study of participants with obstructive de novo or restenotic native upper extremity arteriovenous dialysis fistula lesions treated with drug-coated balloon (DCBs) or standard percutaneous transluminal angioplasty (PTA) following successful high-pressure PTA.
Materials and methods: Participants at 29 international sites were randomized 1:1 to receive an IN.PACT AV DCB (n = 170) or undergo PTA (n = 160). The outcomes through 36 months included target lesion primary patency (TLPP) and access circuit primary patency (ACPP) (composites of clinically driven target lesion or access circuit revascularization and/or access circuit thrombosis), number of reinterventions, and serious adverse events involving the access circuit.
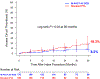
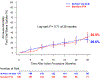
Results: TLPP was 52.1% in the DCB group compared with 36.7% in the PTA group through 24 months and 43.1% in the DCB group compared with 28.6% in the PTA group through 36 months (both log-rank P < .001). ACPP was 39.4% in the DCB group compared with 25.3% in the PTA group through 24 months and 26.4% in the DCB group compared with 16.6% in the PTA group through 36 months (both log-rank P < .001). Cumulative incidence of access circuit thrombosis through 36 months was 8.2% in the DCB group compared with 18.3% in the PTA group (log-rank P = .040). Cumulative incidence of mortality through 36 months was 26.6% in the DCB group compared with 30.8% in the PTA group (log-rank P = .71).
Conclusions: This study demonstrated superior TLPP and ACPP with DCBs compared with PTA, with no difference in mortality through 3 years. Access circuit thrombosis was statistically significantly higher in the PTA group at 3 years.
Copyright © 2023 SIR. Published by Elsevier Inc. All rights reserved.
Figures
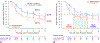


References
-
- United States Renal Data System. 2022 USRDS annual data report: epidemiology of kidney disease in the United States, 2022. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD. Available at: https://adr.usrds.org/2022. Accessed October 3, 2022.
-
- Lok CE, Huber TS, Lee T, et al. KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis 2020; 75:S1–S164. - PubMed
-
- Yuan Y, Cheng W, Lu H. Drug-eluting balloon versus plain balloon angioplasty for the treatment of failing hemodialysis access: a systematic review and meta-analysis. Ann Vasc Surg 2020; 64: 389–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

