Molecular mechanism of GPCR spatial organization at the plasma membrane
- PMID: 37460675
- PMCID: PMC10792125
- DOI: 10.1038/s41589-023-01385-4
Molecular mechanism of GPCR spatial organization at the plasma membrane
Abstract
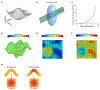
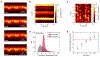
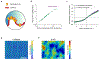
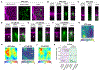
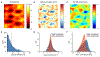
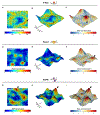
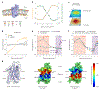
G-protein-coupled receptors (GPCRs) mediate many critical physiological processes. Their spatial organization in plasma membrane (PM) domains is believed to encode signaling specificity and efficiency. However, the existence of domains and, crucially, the mechanism of formation of such putative domains remain elusive. Here, live-cell imaging (corrected for topography-induced imaging artifacts) conclusively established the existence of PM domains for GPCRs. Paradoxically, energetic coupling to extremely shallow PM curvature (<1 µm-1) emerged as the dominant, necessary and sufficient molecular mechanism of GPCR spatiotemporal organization. Experiments with different GPCRs, H-Ras, Piezo1 and epidermal growth factor receptor, suggest that the mechanism is general, yet protein specific, and can be regulated by ligands. These findings delineate a new spatiomechanical molecular mechanism that can transduce to domain-based signaling any mechanical or chemical stimulus that affects the morphology of the PM and suggest innovative therapeutic strategies targeting cellular shape.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
D.S. is the founder of Atomos Biotech. The other authors declare no competing interests.
Figures


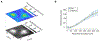


Similar articles
-
Membrane curvature regulates ligand-specific membrane sorting of GPCRs in living cells.Nat Chem Biol. 2017 Jul;13(7):724-729. doi: 10.1038/nchembio.2372. Epub 2017 May 8. Nat Chem Biol. 2017. PMID: 28481347
-
Interplay between G protein-coupled receptors and nanotechnology.Acta Biomater. 2023 Oct 1;169:1-18. doi: 10.1016/j.actbio.2023.07.049. Epub 2023 Jul 28. Acta Biomater. 2023. PMID: 37517621 Review.
-
G protein-coupled receptors function as cell membrane receptors for the steroid hormone 20-hydroxyecdysone.Cell Commun Signal. 2020 Sep 9;18(1):146. doi: 10.1186/s12964-020-00620-y. Cell Commun Signal. 2020. PMID: 32907599 Free PMC article. Review.
-
7TM Domain Structure of Adhesion GPCRs.Handb Exp Pharmacol. 2016;234:43-66. doi: 10.1007/978-3-319-41523-9_3. Handb Exp Pharmacol. 2016. PMID: 27832483 Review.
-
Regulation of G protein-coupled receptor signaling by plasma membrane organization and endocytosis.Traffic. 2019 Feb;20(2):121-129. doi: 10.1111/tra.12628. Epub 2019 Jan 11. Traffic. 2019. PMID: 30536564 Free PMC article. Review.
Cited by
-
Protein-membrane interactions: sensing and generating curvature.Trends Biochem Sci. 2024 May;49(5):401-416. doi: 10.1016/j.tibs.2024.02.005. Epub 2024 Mar 19. Trends Biochem Sci. 2024. PMID: 38508884 Free PMC article. Review.
-
Cell swelling enhances ligand-driven β-adrenergic signaling.Nat Commun. 2024 Sep 7;15(1):7822. doi: 10.1038/s41467-024-52191-y. Nat Commun. 2024. PMID: 39242606 Free PMC article.
-
Investigations of membrane protein interactions in cells using fluorescence microscopy.Curr Opin Struct Biol. 2024 Jun;86:102816. doi: 10.1016/j.sbi.2024.102816. Epub 2024 Apr 21. Curr Opin Struct Biol. 2024. PMID: 38648680 Free PMC article. Review.
-
Higher-order transient membrane protein structures.Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2421275121. doi: 10.1073/pnas.2421275121. Epub 2024 Dec 31. Proc Natl Acad Sci U S A. 2025. PMID: 39739811 Free PMC article.
-
Cellular Signaling at the Nano-Bio Interface: Spotlighting Membrane Curvature.Annu Rev Phys Chem. 2025 Apr;76(1):251-277. doi: 10.1146/annurev-physchem-090722-021151. Annu Rev Phys Chem. 2025. PMID: 40258240 Review.
References
-
- Pierce KL, Premont RT & Lefkowitz RJ Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol 3, 639–650 (2002). - PubMed
-
- Wootten D, Christopoulos A, Marti-Solano M, Babu MM & Sexton PM Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol 19, 638–653 (2018). - PubMed
-
- Nikolaev VO et al. β2-Adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science 327, 1653–1657 (2010). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

