Poorer aging trajectories are associated with elevated serotonin synthesis capacity
- PMID: 37460847
- PMCID: PMC10792105
- DOI: 10.1038/s41380-023-02177-x
Poorer aging trajectories are associated with elevated serotonin synthesis capacity
Abstract
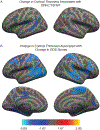
The dorsal raphe nucleus (DRN) is one of the earliest targets of Alzheimer's disease-related tau pathology and is a major source of brain serotonin. We used [18F]Fluoro-m-tyrosine ([18F]FMT) PET imaging to measure serotonin synthesis capacity in the DRN in 111 healthy adults (18-85 years-old). Similar to reports in catecholamine systems, we found elevated serotonin synthesis capacity in older adults relative to young. To establish the structural and functional context within which serotonin synthesis capacity is elevated in aging, we examined relationships among DRN [18F]FMT net tracer influx (Ki) and longitudinal changes in cortical thickness using magnetic resonance imaging, longitudinal changes in self-reported depression symptoms, and AD-related tau and β-amyloid (Aβ) pathology using cross-sectional [18F]Flortaucipir and [11C]Pittsburgh compound-B PET respectively. Together, our findings point to elevated DRN [18F]FMT Ki as a marker of poorer aging trajectories. Older adults with highest serotonin synthesis capacity showed greatest temporal lobe cortical atrophy. Cortical atrophy was associated with increasing depression symptoms over time, and these effects appeared to be strongest in individuals with highest serotonin synthesis capacity. We did not find direct relationships between serotonin synthesis capacity and AD-related pathology. Exploratory analyses revealed nuanced effects of sex within the older adult group. Older adult females showed the highest DRN synthesis capacity and exhibited the strongest relationships between entorhinal cortex tau pathology and increasing depression symptoms. Together these findings reveal PET measurement of the serotonin system to be a promising marker of aging trajectories relevant to both AD and affective changes in older age.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
CONFLICTS OF INTEREST
Dr. Jagust has served as a consultant for Biogen and Bioclinica and holds equity interest in Optoceutics. There are no other financial disclosures.
Figures


References
-
- Andrés-Benito P, Fernández-Dueñas V, Carmona M, Escobar LA, Torrejón-Escribano B, Aso E, et al. Locus coeruleus at asymptomatic early and middle Braak stages of neurofibrillary tangle pathology. Neuropathol Appl Neurobiol. 2017. Aug;43(5):373–92. - PubMed
-
- Parvizi J, Van Hoesen GW, Damasio A. The selective vulnerability of brainstem nuclei to Alzheimer’s disease. Ann Neurol. 2001;49(1):53–66. - PubMed
-
- Rüb U, Stratmann K, Heinsen H, Del Turco D, Seidel K, den Dunnen W, et al. The Brainstem Tau Cytoskeletal Pathology of Alzheimer’s Disease: A Brief Historical Overview and Description of its Anatomical Distribution Pattern, Evolutional Features, Pathogenetic and Clinical Relevance. Curr Alzheimer Res. 2016. Oct 1;13(10):1178–97. - PubMed

