Establishment of induced pluripotent stem cells derived from patients and healthy siblings of a nevoid basal cell carcinoma syndrome family
- PMID: 37460876
- PMCID: PMC10374668
- DOI: 10.1007/s11626-023-00778-y
Establishment of induced pluripotent stem cells derived from patients and healthy siblings of a nevoid basal cell carcinoma syndrome family
Abstract
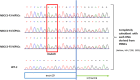
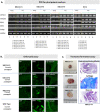
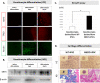
It is known that a nevoid basal cell carcinoma syndrome (NBCCS) is characterized by a combination of developmental abnormalities and a predisposition to form various tumors. Although it is possible to create disease models via gene editing, there are significant potential problems with this approach such as off-target mutations and differences in SNPs. On the other hand, since disease families share common SNPs, research using iPSCs derived from both patients and healthy siblings of the same disease family is very important. Thus, establishment of induced pluripotent stem cells derived from patients and healthy siblings of the same NBCCS family will be of great importance to study the etiology of this disease and to develop therapeutics. In this study, we generated hiPSCs using peripheral blood mononuclear cells derived from the patients and healthy siblings of familial NBCCS with the novel mutation in PTCH1_c.3298_3299insAAG in the feeder- and serum-free culture conditions using SeVdp. In addition, disease-specific hiPSCs such as those expressing the PTCH1_c.3298_3299insAAG mutation could be powerful tools for revealing the genotype-phenotype relationship and pathogenicity of NBCCS.
© 2023. The Author(s).
Figures
Similar articles
-
Somatic mosaicism containing double mutations in PTCH1 revealed by generation of induced pluripotent stem cells from nevoid basal cell carcinoma syndrome.J Med Genet. 2017 Aug;54(8):579-584. doi: 10.1136/jmedgenet-2016-104490. Epub 2017 Mar 31. J Med Genet. 2017. PMID: 28363938
-
Whole-exome sequencing of nevoid basal cell carcinoma syndrome families and review of Human Gene Mutation Database PTCH1 mutation data.Mol Genet Genomic Med. 2018 Nov;6(6):1168-1180. doi: 10.1002/mgg3.498. Epub 2018 Nov 8. Mol Genet Genomic Med. 2018. PMID: 30411536 Free PMC article.
-
PTCH1 Germline Mutations and the Basaloid Follicular Hamartoma Values in the Tumor Spectrum of Basal Cell Carcinoma Syndrome (NBCCS).Anticancer Res. 2018 Jan;38(1):471-476. doi: 10.21873/anticanres.12246. Anticancer Res. 2018. PMID: 29277811
-
Nevoid basal cell carcinoma syndrome (Gorlin syndrome).Orphanet J Rare Dis. 2008 Nov 25;3:32. doi: 10.1186/1750-1172-3-32. Orphanet J Rare Dis. 2008. PMID: 19032739 Free PMC article. Review.
-
[Skeletal and dermatological manifestations of the nevoid Basal cell carcinoma syndrome (Gorlin-Goltz syndrome). Results of 8 patients in 12 years].Rofo. 2007 Jun;179(6):618-26. doi: 10.1055/s-2007-963117. Epub 2007 May 9. Rofo. 2007. PMID: 17492539 Review. German.
References
-
- Endo M, Fujii K, Sugita K, Saito K, Kohno Y, Miyashita T (2012) Nationwide survey of nevoid basal cell carcinoma syndrome in Japan revealing the low frequency of basal cell carcinoma. Am J Med Genet A 158a: 351–357. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

