Helicobacter pylori-activated fibroblasts as a silent partner in gastric cancer development
- PMID: 37460910
- PMCID: PMC10713772
- DOI: 10.1007/s10555-023-10122-1
Helicobacter pylori-activated fibroblasts as a silent partner in gastric cancer development
Abstract
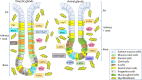
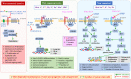
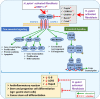
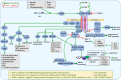
The discovery of Helicobacter pylori (Hp) infection of gastric mucosa leading to active chronic gastritis, gastroduodenal ulcers, and MALT lymphoma laid the groundwork for understanding of the general relationship between chronic infection, inflammation, and cancer. Nevertheless, this sequence of events is still far from full understanding with new players and mediators being constantly identified. Originally, the Hp virulence factors affecting mainly gastric epithelium were proposed to contribute considerably to gastric inflammation, ulceration, and cancer. Furthermore, it has been shown that Hp possesses the ability to penetrate the mucus layer and directly interact with stroma components including fibroblasts and myofibroblasts. These cells, which are the source of biophysical and biochemical signals providing the proper balance between cell proliferation and differentiation within gastric epithelial stem cell compartment, when exposed to Hp, can convert into cancer-associated fibroblast (CAF) phenotype. The crosstalk between fibroblasts and myofibroblasts with gastric epithelial cells including stem/progenitor cell niche involves several pathways mediated by non-coding RNAs, Wnt, BMP, TGF-β, and Notch signaling ligands. The current review concentrates on the consequences of Hp-induced increase in gastric fibroblast and myofibroblast number, and their activation towards CAFs with the emphasis to the altered communication between mesenchymal and epithelial cell compartment, which may lead to inflammation, epithelial stem cell overproliferation, disturbed differentiation, and gradual gastric cancer development. Thus, Hp-activated fibroblasts may constitute the target for anti-cancer treatment and, importantly, for the pharmacotherapies diminishing their activation particularly at the early stages of Hp infection.
Keywords: Cancer-associated fibroblasts; Cell reprogramming; Cell signaling; Gastric cancer; Helicobacter pylori–induced fibroblast activation; Stemness.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Involvement of epithelial-mesenchymal transition-inducing transcription factors in the mechanism of Helicobacter pylori-induced fibroblasts activation.J Physiol Pharmacol. 2019 Oct;70(5). doi: 10.26402/jpp.2019.5.08. Epub 2019 Dec 26. J Physiol Pharmacol. 2019. PMID: 31889044
-
Helicobacter pylori-activated gastric fibroblasts induce epithelial-mesenchymal transition of gastric epithelial cells in vitro in a TGF-β-dependent manner.Helicobacter. 2019 Oct;24(5):e12653. doi: 10.1111/hel.12653. Epub 2019 Aug 14. Helicobacter. 2019. PMID: 31411795
-
Long-Term Helicobacter pylori Infection Switches Gastric Epithelium Reprogramming Towards Cancer Stem Cell-Related Differentiation Program in Hp-Activated Gastric Fibroblast-TGFβ Dependent Manner.Microorganisms. 2020 Oct 2;8(10):1519. doi: 10.3390/microorganisms8101519. Microorganisms. 2020. PMID: 33023180 Free PMC article.
-
[Progress on the effects of cancer-related fibroblast induced by Helicobacter pylori infection on gastric epithelial-mesenchymal transition].Sheng Li Xue Bao. 2024 Aug 25;76(4):547-560. Sheng Li Xue Bao. 2024. PMID: 39192788 Review. Chinese.
-
Chemoprevention of gastric cancer by Helicobacter pylori eradication and its underlying mechanism.J Gastroenterol Hepatol. 2019 Aug;34(8):1287-1295. doi: 10.1111/jgh.14646. Epub 2019 Mar 27. J Gastroenterol Hepatol. 2019. PMID: 30828872 Review.
Cited by
-
Cancer Development and Progression Through a Vicious Cycle of DNA Damage and Inflammation.Int J Mol Sci. 2025 Apr 3;26(7):3352. doi: 10.3390/ijms26073352. Int J Mol Sci. 2025. PMID: 40244228 Free PMC article. Review.
-
Autonomic nervous system development-related signature as a novel predictive biomarker for immunotherapy in pan-cancers.Front Immunol. 2025 Jul 23;16:1611890. doi: 10.3389/fimmu.2025.1611890. eCollection 2025. Front Immunol. 2025. PMID: 40771813 Free PMC article.
-
Inflammatory response in gastrointestinal cancers: Overview of six transmembrane epithelial antigens of the prostate in pathophysiology and clinical implications.World J Clin Oncol. 2024 Jan 24;15(1):9-22. doi: 10.5306/wjco.v15.i1.9. World J Clin Oncol. 2024. PMID: 38292664 Free PMC article. Review.
-
Identification of multiomics and immune infiltration-associated biomarkers for early gastric cancer: a machine learning-based diagnostic model development study.BMC Cancer. 2025 May 31;25(1):972. doi: 10.1186/s12885-025-14396-2. BMC Cancer. 2025. PMID: 40450287 Free PMC article.
-
Six transmembrane epithelial antigens of the prostate to illustrate inflammatory response in gastrointestinal cancers.World J Clin Oncol. 2024 Aug 24;15(8):961-964. doi: 10.5306/wjco.v15.i8.961. World J Clin Oncol. 2024. PMID: 39193158 Free PMC article.
References
-
- Robinson, K., Argent, R. H., & Atherton, J. C. (2007). The inflammatory and immune response to Helicobacter pylori infection. Best Practice and Research: Clinical Gastroenterology, 21(2). 10.1016/j.bpg.2007.01.001 - PubMed
-
- Konturek, P. C., Konturek, S. J., Brzozowski, T. (2009). Helicobacter pylori infection in gastric cancerogenesis. Journal of Physiology and Pharmacology,60(3), 3–21. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

