Targeting miRNA by CRISPR/Cas in cancer: advantages and challenges
- PMID: 37460924
- PMCID: PMC10351202
- DOI: 10.1186/s40779-023-00468-6
Targeting miRNA by CRISPR/Cas in cancer: advantages and challenges
Abstract
Clustered regulatory interspaced short palindromic repeats (CRISPR) has changed biomedical research and provided entirely new models to analyze every aspect of biomedical sciences during the last decade. In the study of cancer, the CRISPR/CRISPR-associated protein (Cas) system opens new avenues into issues that were once unknown in our knowledge of the noncoding genome, tumor heterogeneity, and precision medicines. CRISPR/Cas-based gene-editing technology now allows for the precise and permanent targeting of mutations and provides an opportunity to target small non-coding RNAs such as microRNAs (miRNAs). However, the development of effective and safe cancer gene editing therapy is highly dependent on proper design to be innocuous to normal cells and prevent introducing other abnormalities. This study aims to highlight the cutting-edge approaches in cancer-gene editing therapy based on the CRISPR/Cas technology to target miRNAs in cancer therapy. Furthermore, we highlight the potential challenges in CRISPR/Cas-mediated miRNA gene editing and offer advanced strategies to overcome them.
Keywords: CRISPR; CRISPR/Cas12; CRISPR/Cas9; Cancer therapy; Gene editing; miRNAs.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



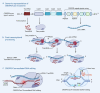

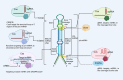


References
-
- Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol. 1987;169(12):5429–5433. doi: 10.1128/jb.169.12.5429-5433.1987. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

